Excel at Science
- Feb 16, 2021

AP Biology Past FRQs by Topic
Updated: Jan 31

**Updated on 1/31/24 to include the 2022-23 FRQ exams!**
If you are looking for past AP Biology free-response questions (FRQs) that are organized by topic, then you have come to the right place. In this post, we have linked every freely available past FRQ there is from College Board and organized it into the following major topics of AP Biology .
(Please note that we are not associated with College Board and are simply sharing the resources they have made available to students.)
Biochemistry
Metabolism & energetics.
Physiology (note that this topic will not be tested on the official AP Biology exam this year in 2021, although many questions about physiology could also cover concepts that will be tested)
Experiment design & data analysis
Need more AP-style practice problems?
Intensively doing and reviewing practice questions is proven to be much more effective than spending hours studying. Check out our AP Bio Practice Portal , which is an easy-to-use database of 300+ AP-style MCQ and FRQ practice questions. Students love the Practice Portal because it includes answers and explanations for every problem, tracks progress, and saves time from Googling practice problems.
Try the Practice Portal >
How to make the most of past frqs from college board.
As noted above, the diversity of organisms, plants, and physiology will not be on the 2021 AP Biology exam. However, the exam could include questions about topics or hypothetical situations that are related to those topics. One great example is cell communication, which is used in multiple systems inside our bodies. Let’s say an FRQ was to appear about the immune system and how the immune cells communicate. That would be fair game as long as the question focuses on the cell signaling part, not the details of the immune system. If the question requires some background knowledge about the immune system, it will be provided.
If you want to do a whole practice FRQ set just like the ones on the real exam (which we highly recommend), all the freely available past FRQs by year are available here on the College Board website. Tip: time yourself and take the practice FRQ set in an environment that mimics how you imagine your actual testing environment to be.
If you would like to focus on a particular topic, then the section coming up is for you. Some FRQs will show up under multiple topics because they truly do test students’ understanding of multiple different topics.
Tip : Whether you are doing individual free-response questions or doing a full problem set in one go, it is extremely important and effective to do test corrections! Don’t only consult the scoring guidelines and model responses when you have no clue how to answer a question. You should be checking them for all the FRQs you do. When you find a difference between your answer and the scoring guidelines, it is important that you pause and analyze why your response is incorrect. Take the time to understand your mistakes and see how your answer could have been better. This will help you boost your scores the most efficiently.
AP BIOLOGY FRQs BY TOPIC
Below are the linked FRQs organized by topic. The header for each topic will also lead you to the corresponding study guide that will help you review the unit in detail!
Basic and organic chemistry concepts do not come up often on the FRQs (but of course, it’s better to be prepared). The properties of water and macromolecules come up occasionally.
2017 #7 and 8
Includes cell structure and function, cell transport and the proteins involved.
2019 #3 and 8
2018 #2, 6, and 8
2006 #1, 3, and 4
2001 #1 and 4
(study guide coming soon!)
This unit includes enzymes, cellular respiration, and photosynthesis.
2023 #2 (cell respiration & photosynthesis)
2023 #4 (photosynthesis)
2022 #3 (enzymes)
2021 #3 (cell respiration)
2019 #3 (cell respiration)
2018 #2 (cell respiration)
2017 #7 (cell respiration)
2017 #5 (photosynthesis)
2015 #2 (cell respiration)
2013 #2 (photosynthesis) and 4 (cell respiration & photosynthesis)
2012 #2 (cell respiration) and 4 (cell respiration & photosynthesis)
2010 #2 (enzymes)
2007 #3 (photosynthesis)
2006 #4 (photosynthesis)
2005 #1 (cell respiration & photosynthesis)
2004 #3 (photosynthesis)
Cell cycle & cell signaling
This topic has shown up more frequently and in more difficult FRQs in recent years, especially cell communication. The trend will most likely continue so definitely prioritize reviewing and practicing this topic!
2023 #1 (cell communication)
2022 #1 (cell communication)
2022 #2 (cell cycle, meiosis)
2021 #1 (cell communication)
2019 #4 (cell communication)
2018 #8 (cell communication)
2017 #8 (cell communication)
2016 # 7 (cell division)
2015 # 4 (cell division)
2015 #5 and 7 (cell communication)
2013 #8 (cell communication)
2011 #1B (cell division)
2010 #1 (cell communication)
2006 #1B (cell division)
2004 #1 (cell division)
Genetics, Gene Expression and Regulation
Genetics Pt 1 and Genetics Pt 2 Study Guides
This section includes the classic Mendelian genetics, with Punnett squares, crosses, and Mendel’s laws. It also includes DNA replication, protein synthesis, and gene expression regulation for both eukaryotes and prokaryotes.
2023 #6 (gene expression)
2022 #6 (protein synthesis, gene expression)
2021 #6 (gene expression)
2021 #2 (heredity + pedigrees)
2020 #1 parts a-b
2019 #1 and 3
2018 #1, 4, and 7
2016 #4 and 7
2023 #5 (Cladistics)
2022 #4 (speciation)
2020 #1 parts f-j
2015 #3 and 6
2014 #2 and 4
2015 #2 (nervous system)
2014 #2 (immune system) and 6 (musculoskeletal system) and 7
2017 #2, 4, and 7b
2016 #3 and 5
2014 #3 and 4
Experimental design & analysis
This is an additional section that isn’t focused on any particular topic or has significant data analysis involved. While most FRQs do pertain to a specific topic(s), some are simply there to test your knowledge of experimental design and understanding of statistical concepts such as performing Chi-Square tests and interpreting error bars on graphs. These types of questions have become more and more common on the AP exam, so it is important to feel comfortable and confident with them.
2023 #6 (data analysis)
2022 #3 (experiment design)
2020 #1 parts c-e
2016 #2 , 6 and 8
2014 #1 and 5
2013 #1 and 7
Hope these organized FRQs saved you some time so you can focus more on actually doing them and practicing! You can easily share this post with friends who may find it helpful as well.
How to Improve AP Biology FRQ Scores, Fast
Do a lot of FRQ practice problems and review the answers! Practice is key, especially for a subject as dense as AP Bio. Check out the AP Bio Practice Portal , which is our popular vault of 300+ AP-style MCQ and FRQ problem sets with answers and explanations for every question. Don't waste any more time Googling practice problems or answers - try it out now!
Recent Posts
How to Study for AP Biology Finals: Tactical Strategies for Success
How to Interpret Diagrams and Graphs on AP Biology Exams
How to Get a 5 on the AP Biology Exam: A Comprehensive Study Guide
ความคิดเห็น

AP® Biology
Enzymes: ap® biology crash course review.
- The Albert Team
- Last Updated On: March 1, 2022
In this AP® Biology Crash Course , we will review what you need to know about enzymes for the AP® Biology exam. We will cover what enzymes are, how enzymes work, some factors that affect how they work, and finally an example of an AP® Bio question about enzymes.
What are Enzymes?
Enzymes are proteins that catalyze chemical reactions. Molecules at the beginning of the chemical reactionary process are called substrates, and these are converted into products. Enzyme kinetics, or Michaelis-Menten kinetics, investigate how enzymes bind substrates and turn them into products. The amount of substrate needed to reach a given rate of reaction is the Michaelis-Menten constant. Almost all metabolic processes require enzymes to occur at the proper rate.
Some chemical reactions take a lot of energy to start. The amount of energy needed to kick off a chemical reaction is called its activation energy . Enzymes help the chemical reaction reach the activation energy by lowering the amount of energy needed to overcome it.
French chemist Anselme Payen discovered the first recognized enzyme, diastase , in 1833. Louis Pasteur also noticed when studying a mixture of sugar, alcohol, and yeast, something was happening to ignite the fermentation process. The word “enzyme” was first used by a German physiologist in 1877 named Wilhelm Kuhne.
Enzyme Structure
As you may have learned in your AP® Biology course , an enzyme’s primary structure is nothing more than a long sequence of amino acids that bond with one another. Short-range interactions ( secondary ) between amino acids can be alpha-helix or beta sheet. Alphas look like spirals, and betas look like flat, wavy sheets.
The long-range interactions ( tertiary ) are when amino acids interact with other amino acids a long way down the strand, and as they fold over, they form a globular structure. The quaternary structure is when one globular strand interacts with other tertiary pieces. When bonds are formed at this level, they are often hydrogen bonds, but sometimes it is two hydrophobic pieces interacting, or even ionic bonds. Alternatively, when an enzyme is unfolded, it’s referred to as being denatured.
Enzymes are quite large relative to their substrates, yet only a small portion of the structure is involved in the reaction; that part is referred to as the catalytic site . This site is located next to a binding site where residues orient the substrates. These two sites together are referred to as the active site .
Enzyme Activation
In order for an enzyme to work, it must be activated by the binding of another molecule. Activators can either be cofactors or coenzymes ; cofactors are small, inorganic chemicals, and coenzymes are organic compounds. Both of these activators bind to the active site but are not considered substrates. When they bind to the active site, there is often a conformation change. A conformation change is a change in the enzyme’s configuration or shape. The change in shape alters the active site and allows the substrate to bind.
How Do Enzymes Work?
Enzymes are extremely selective about which substrates they are able to bind to. Related to the specificity of enzyme and substrate bonding, Emil Fischer proposed the lock and key model where the two would have complementary geometric forms. Daniel Koshland suggested that these complementary geometric pieces can actually shift and can even be reshaped by their interactions with substrates. This new discovery led to the induced fit model .
The induced fit model refers to the ability for the substrate and enzyme to modify their shape in order to fit together. After the enzyme and substrate have bound to each other, the enzyme will work to lower the activation energy of the chemical reaction.
In order to understand how enzymes work, we should review activation energy and Gibbs free energy. Using the Gibbs free energy models, we can see that the energy of the reactants is lower than the activation energy. The activation energy (delta G) is the amount of energy that is needed to make this reaction move forward. When the reaction is catalyzed by an enzyme, the amount of activation is greatly reduced, making that hump easier for the reactants to get over.
Enzymes are able to lower the activation energy of a chemical reaction by making changes to the transition state of the reaction. By stabilizing the transition state, the reaction will move toward the transition state more easily. Without an enzyme, the transition state is often not energetically favorable. The enzyme will alter the transition state in order to make it more favorable and to move the reaction forward. Similarly, the enzyme can lower the energy of the transition state, which will allow the reaction to move forward.
Inhibitors bind to an enzyme to decrease its activity. The prevention of substrate-enzyme binding is a form of regulation. Negative feedback is an example of a time when inhibitors are important. If the body has produced too much of the final products of a reaction, those final products can feedback to the reaction and prevent the enzyme and substrate from binding. In essence, in negative feedback, the end products are telling the body to stop creating them.
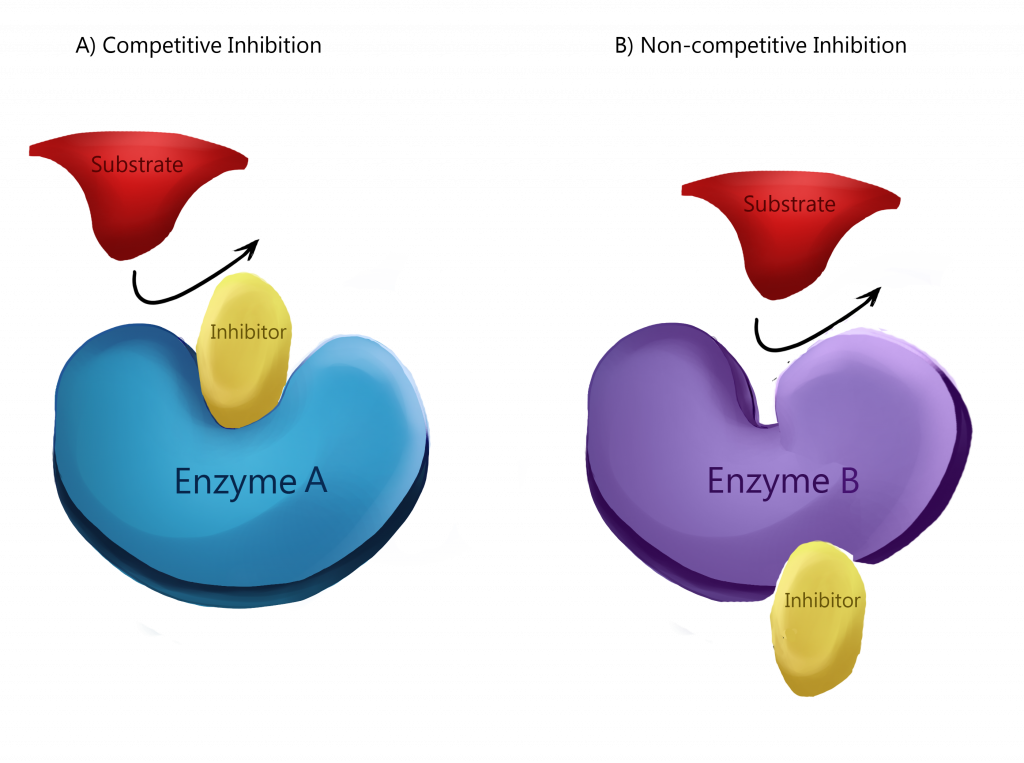
There are two types of inhibition that are used for regulation, competitive and non-competitive . In competitive inhibition, the inhibitor binds directly to the active site, effectively completely blocking access from the substrate.
Non-competitive inhibition, also known as allosteric inhibition , is when the inhibitor binds to a different part of the enzyme but induces a change in the active site to prevent binding by the substrate. The binding often changes the shape or charge of the binding site, preventing the substrate from being able to bind. The other way to inhibit is to bond. These processes all help to regulate rates of enzyme activity.
Factors that Affect Enzyme Activity
Enzyme activity is affected by many factors, including temperature and pH . An increase in temperature increases the rate at which the molecules in a system move. This increase in temperature will allow the substrates and enzymes to locate each other more quickly. However, there is a point at which the enzyme will become denatured due to the higher temperature, adding stress to its bonds. Many enzymes operate at an ideal temperature called the optimum temperature .
pH can also affect an enzymes activity. pH controls the balance between positively and negatively charged amino acids. Ionic interactions are important to hold the enzymes together. Most enzymes have an optimum pH between 6 and 8.
Now that we have covered the topic of enzymes, let’s explore a real life example. At this point in your studies, you may have come across an enzyme called DNA polymerase (if you haven’t please check out AP® Biology Crash Course Review: DNA Replication ). DNA polymerase is an enzyme that catalyzes the chemical reaction of deoxynucleoside triphosphate plus DNA to diphosphate and DNA (plus the nucleotide).
In this reaction, the enzyme breaks a phosphate bond from the deoxynucleoside triphosphate and uses that energy to add the nucleotide base to the DNA molecule. Without DNA polymerase, this process would not be able to occur because it is energetically unfavorable to catalyze. If this process could not occur, our cells would not be able to replicate and repair. This would result in death of the organism.
AP® Biology Question
Now that we have reviewed the information you need to know about enzymes for the AP® Biology exam, here is an example of a multiple choice question you could see:
Which of the following is characteristic of enzymes?
A. They lower the energy of activation of a reaction by binding the substrate.
B. They raise the energy of activation of a reaction by binding the substrate.
C. They lower the amount of energy present in the substrate.
D. They raise the amount of energy present in the substrate.
What did you pick? If you chose A you are correct! Enzymes lower activation energy when they bind to the substrate and alter the transition state. If you had trouble with this question, go back through and read this review. If you have any questions, let us know in the comment section!
In nearly every chemical reaction of life, enzymes are used. Dr. Richard Wolfenden recently found that if enzymes were removed, the biological reactions necessary to life would take 2.3 billion years to spontaneously occur. Clearly, enzymes are a necessary part of life!
In this AP® Biology Crash Course Review , we went over the general structure of an enzyme and its activation site. We then reviewed what exactly enzymes do and how they do it. We then reviewed different types of activation and inhibition molecules. Finally, we wrapped up with an example of a real life enzyme and why it is important to survival.
The AP® Bio exam will likely have questions about enzymes on it. Do you feel prepared? Let us know!
Need help preparing for your AP® Biology exam?
Albert has hundreds of AP® Biology practice questions, free response, and full-length practice tests to try out.
Interested in a school license?
Popular posts.

AP® Score Calculators
Simulate how different MCQ and FRQ scores translate into AP® scores

AP® Review Guides
The ultimate review guides for AP® subjects to help you plan and structure your prep.

Core Subject Review Guides
Review the most important topics in Physics and Algebra 1 .

SAT® Score Calculator
See how scores on each section impacts your overall SAT® score

ACT® Score Calculator
See how scores on each section impacts your overall ACT® score

Grammar Review Hub
Comprehensive review of grammar skills

AP® Posters
Download updated posters summarizing the main topics and structure for each AP® exam.
Interested in a school license?

Bring Albert to your school and empower all teachers with the world's best question bank for: ➜ SAT® & ACT® ➜ AP® ➜ ELA, Math, Science, & Social Studies aligned to state standards ➜ State assessments Options for teachers, schools, and districts.
AP CENTRAL FRQ's 2023 2022-1999 2020 Exam questions available in AP Classroom question bank
2017 #3 Free-Response Questions Scoring Guidelines Sample Responses Q3 Use codon chart to predict mutation Impact of amino acid substitution Mutations Phenotype/genotype
2010 #2 2010 All Questions RUBRIC Sample Responses Q2 Amylase Enzyme Lab Make a graph from data provided Calculate reaction rate Explain changes in graph over time Make a graph prediction about doubling amylase concentration Explain the effect of environmental factors on enzyme activity
2005 #1 2005 All Questions Scoring Guidelines Sample Responses Q1 Yeast respiration LAB Draw graph Determine optimum temperature Impact of temperature on enzymes Design experiment Predict results
2000 #1 2000 All Questions Scoring Guidelines Sample Responses Q1 Effect of temperature/pH on enzyme activity Enzyme structure/function Describe experiment State hypothesis
OLD FRQ's prior to 1995
(1994) Enzymes are biological catalysts. a. Relate the chemical structure of an enzyme to its specificity and catalytic activity. b. Design a quantitative experiment to investigate the influence of pH or temperature on the activity of an enzyme. c. Describe what information concerning the structure of an enzyme could be inferred from your experiment .
(1981) Discuss the biological importance of each of the following organic compounds in relation to cellular structure and function in plants and animals. a. Carbohydrates b. Proteins c. Lipids d. Nucleic Acids
(1978) Give specific examples to illustrate the theory of enzyme action, and include in your discussion the effects of each of the following: a. Substrate concentration b. pH shifts c. Temperature shifts d. Competitive inhibition.
(1975) Describe the chemical compositions and configuration of enzymes and discuss the factors that modify enzyme structure and/or function.
(1973) Hypotheses derived from laboratory experiments and field observations have been advanced to explain the origin of life on earth. Starting with a probable prelife environment, describe the formation and evolution of the various trophic forms (nutrition types) to and including unicellular organisms. Describe at least one experiment whose results support one of these scientific hypotheses.
(1972) A class of biology students performed an experiment on the digestion of starch by salivary amylase. Each student determined the length of time required for different dilutions of his saliva to digest completely a standard concentration of starch. Iodine was used to test for the presence of starch. The results obtained by some of the class are summarized in the table below.
TIME REQUIRED FOR THE DISAPPEARANCE OF STARCH WITH VARIOUS SALIVA DILUTIONS(saliva: H 2 O)
Student 1:9 (10%) 1:19 (5%) 1:49 (2%) 1:99 (1%)
A 45 seconds 50 seconds 100 seconds 135 seconds B (no end point) C 90 seconds 100 seconds 200 seconds 270 seconds D 260 seconds 300 seconds 600 seconds 800 seconds
a. Present the data for Student A in graphic form. b. Carefully examine the data collected by the four students above and state as many conclusions as you can that are supported by these data. c. Assuming there have been no errors in techniques, form as many hypotheses as you can to explain the differences observed. d. Design one experiment to test the validity of one hypothesis. Clearly state what data you would want to collect in this experiment to test your hypothesis.
(1969) Proteins functioning as enzymes exhibit precise specifications. Discuss the levels of structural organization within proteins which are responsible for specific molecular interaction.
(1968) Suppose that you have isolated an extract from a tissue and you have found that the extract speeds up the rate of a particular reaction. What kind of information would you need to demonstrate that the substance responsible for increasing the rate of this reaction is an enzyme? Explain how this information would indicate that the catalytic effect is due to an enzyme.
6.5 Enzymes
Learning objectives.
In this section, you will explore the following questions:
- What is the role of enzymes in metabolic pathways?
- How do enzymes function as molecular catalysts?
Connection for AP ® Courses
Many chemical reactions in cells occur spontaneously, but happen too slowly to meet the needs of a cell. For example, a teaspoon of sucrose (table sugar), a disaccharide, in a glass of iced tea will take time to break down into two monosaccharides, glucose and fructose; however, if you add a small amount of the enzyme sucrase to the tea, sucrose breaks down almost immediately. Sucrase is an example of an enzyme, a type of biological catalyst. Enzymes are macromolecules—most often proteins—that speed up chemical reactions by lowering activation energy barriers. Enzymes are very specific for the reactions they catalyze; because they are polypeptides, enzymes can have a variety of shapes attributed to interactions among amino acid R-groups. One part of the enzyme, the active site, interacts with the substrate via the induced fit model of interaction. Substrate binding alters the shape of the enzyme to facilitate the chemical reaction in several different ways, including bringing substrates together in an optimal orientation. After the reaction finishes, the product(s) are released, and the active site returns to its original shape.
Enzyme activity, and thus the rate of an enzyme-catalyzed reaction, is regulated by environmental conditions, including the amount of substrate, temperature, pH, and the presence of coenzymes, cofactors, activators, and inhibitors. Inhibitors, coenzymes, and cofactors can act competitively by binding to the enzyme’s active site, or noncompetitively by binding to the enzyme’s allosteric site. An allosteric site is an alternate part of the enzyme that can bind to non–substrate molecules. Enzymes work most efficiently under optimal conditions that are specific to the enzyme. For example, trypsin, an enzyme in the human small intestine, works most efficiently at pH 8, whereas pepsin in the stomach works best under acidic conditions. Sometimes environmental factors, especially low pH and high temperatures, alter the shape of the active site; if the shape cannot be restored, the enzyme denatures. The most common method of enzyme regulation in metabolic pathways is via feedback inhibition.
How can various factors, such as feedback inhibition, regulate enzyme activity?
Information presented and the examples highlighted in the section support concepts and Learning Objectives outlined in Big Idea 4 of the AP ® Biology Curriculum Framework. The learning objectives listed in the Curriculum Framework provide a transparent foundation for the AP ® Biology course, an inquiry-based laboratory experience, instructional activities, and AP ® Exam questions. A Learning Objective merges required content with one or more of the seven science practices.
Teacher Support
The idea that enzymes help chemical reactions to occur, but do not take part in the chemical reaction and are not changed by it can be confusing. Stress that an enzyme and substrate do not covalently bind to each other and the association is temporary. Figure 6.16 is useful in illustrating enzyme function. If two compounds are to be joined into one during the reaction, and they would anyway if left alone long enough, the enzyme molecule brings them close enough for the reaction to occur faster. If a large molecule is to be split into smaller units, the enzyme stresses the molecule and makes it easier for the covalent bonds holding the molecule to break. In both cases, the enzyme molecule subtlety changes its shape after attaching to the substrate (s). This creates an intermediate phase of the reaction and an enzyme-substrate complex. When the reaction is complete and the product(s) disassociate, the enzyme returns to its original shape.
The Science Practice Challenge Questions contain additional test questions for this section that will help you prepare for the AP exam. These questions address the following standards: [APLO 2.15][APLO 4.8][APLO 2.16]
A substance that helps a chemical reaction to occur is a catalyst, and the special molecules that catalyze biochemical reactions are called enzymes. Almost all enzymes are proteins, made up of chains of amino acids, and they perform the critical task of lowering the activation energies of chemical reactions inside the cell. Enzymes do this by binding to the reactant molecules, and holding them in such a way as to make the chemical bond-breaking and bond-forming processes take place more readily. It is important to remember that enzymes don’t change the ∆G of a reaction. In other words, they don’t change whether a reaction is exergonic (spontaneous) or endergonic. This is because they don’t change the free energy of the reactants or products. They only reduce the activation energy required to reach the transition state ( Figure 6.15 ).
Enzyme Active Site and Substrate Specificity
The chemical reactants to which an enzyme binds are the enzyme’s substrates . There may be one or more substrates, depending on the particular chemical reaction. In some reactions, a single-reactant substrate is broken down into multiple products. In others, two substrates may come together to create one larger molecule. Two reactants might also enter a reaction, both become modified, and leave the reaction as two products. The location within the enzyme where the substrate binds is called the enzyme’s active site . The active site is where the “action” happens, so to speak. Since enzymes are proteins, there is a unique combination of amino acid residues (also called side chains, or R groups) within the active site. Each residue is characterized by different properties. Residues can be large or small, weakly acidic or basic, hydrophilic or hydrophobic, positively or negatively charged, or neutral. The unique combination of amino acid residues, their positions, sequences, structures, and properties, creates a very specific chemical environment within the active site. This specific environment is suited to bind, albeit briefly, to a specific chemical substrate (or substrates). Due to this jigsaw puzzle-like match between an enzyme and its substrates (which adapts to find the best fit between the transition state and the active site), enzymes are known for their specificity. The “best fit” results from the shape and the amino acid functional group’s attraction to the substrate. There is a specifically matched enzyme for each substrate and, thus, for each chemical reaction; however, there is flexibility as well.
The fact that active sites are so perfectly suited to provide specific environmental conditions also means that they are subject to influences by the local environment. It is true that increasing the environmental temperature generally increases reaction rates, enzyme-catalyzed or otherwise. However, increasing or decreasing the temperature outside of an optimal range can affect chemical bonds within the active site in such a way that they are less well suited to bind substrates. High temperatures will eventually cause enzymes, like other biological molecules, to denature , a process that changes the natural properties of a substance. Likewise, the pH of the local environment can also affect enzyme function. Active site amino acid residues have their own acidic or basic properties that are optimal for catalysis. These residues are sensitive to changes in pH that can impair the way substrate molecules bind. Enzymes are suited to function best within a certain pH range, and, as with temperature, extreme pH values (acidic or basic) of the environment can cause enzymes to denature.
Induced Fit and Enzyme Function
For many years, scientists thought that enzyme-substrate binding took place in a simple “lock-and-key” fashion. This model asserted that the enzyme and substrate fit together perfectly in one instantaneous step. However, current research supports a more refined view called induced fit ( Figure 6.16 ). The induced-fit model expands upon the lock-and-key model by describing a more dynamic interaction between enzyme and substrate. As the enzyme and substrate come together, their interaction causes a mild shift in the enzyme’s structure that confirms an ideal binding arrangement between the enzyme and the transition state of the substrate. This ideal binding maximizes the enzyme’s ability to catalyze its reaction.
Link to Learning
View an animation of induced fit at this website .
- Production of energy by glycolysis will occur more slowly than normal; skeletal muscles will function properly.
- Production of energy by glycolysis will not occur; skeletal muscles will function properly.
- Production of energy by glycolysis will occur more erratically than normal; skeletal muscles will not function properly.
- Production of energy by glycolysis will not occur; skeletal muscles will not function properly.
When an enzyme binds its substrate, an enzyme-substrate complex is formed. This complex lowers the activation energy of the reaction and promotes its rapid progression in one of many ways. On a basic level, enzymes promote chemical reactions that involve more than one substrate by bringing the substrates together in an optimal orientation. The appropriate region (atoms and bonds) of one molecule is juxtaposed to the appropriate region of the other molecule with which it must react. Another way in which enzymes promote the reaction of their substrates is by creating an optimal environment within the active site for the reaction to occur. Certain chemical reactions might proceed best in a slightly acidic or non-polar environment. The chemical properties that emerge from the particular arrangement of amino acid residues within an active site create the perfect environment for an enzyme’s specific substrates to react.
You’ve learned that the activation energy required for many reactions includes the energy involved in manipulating or slightly contorting chemical bonds so that they can easily break and allow others to reform. Enzymatic action can aid this process. The enzyme-substrate complex can lower the activation energy by contorting substrate molecules in such a way as to facilitate bond-breaking, helping to reach the transition state. Finally, enzymes can also lower activation energies by taking part in the chemical reaction itself. The amino acid residues can provide certain ions or chemical groups that actually form covalent bonds with substrate molecules as a necessary step of the reaction process. In these cases, it is important to remember that the enzyme will always return to its original state at the completion of the reaction. One of the hallmark properties of enzymes is that they remain ultimately unchanged by the reactions they catalyze. After an enzyme is done catalyzing a reaction, it releases its product(s).
Science Practice Connection for AP® Courses
Think about it.
AP Biology Investigation 13: Enzyme Activity. This investigation allows you to design and conduct experiments to explore the effects of environmental variables, such as temperature and pH, on the rates of enzymatic reactions.
This lab investigation is an application of LO 4.17 and Science Practice 5.1 because you will analyze experimental data to determine how various environment conditions affect enzyme structure and function and, thus, the rate of enzyme-catalyzed reactions.
An expanded lab investigation for enzymes, involving determining the effect of pH on the action of turnip peroxidase, is available from the College Board’s ® AP Biology Investigative Labs: An Inquiry-Based Approach , Investigation 13 .
Control of Metabolism Through Enzyme Regulation
It would seem ideal to have a scenario in which all of the enzymes encoded in an organism’s genome existed in abundant supply and functioned optimally under all cellular conditions, in all cells, at all times. In reality, this is far from the case. A variety of mechanisms ensure that this does not happen. Cellular needs and conditions vary from cell to cell, and change within individual cells over time. The required enzymes and energetic demands of stomach cells are different from those of fat storage cells, skin cells, blood cells, and nerve cells. Furthermore, a digestive cell works much harder to process and break down nutrients during the time that closely follows a meal compared with many hours after a meal. As these cellular demands and conditions vary, so do the amounts and functionality of different enzymes.
Since the rates of biochemical reactions are controlled by activation energy, and enzymes lower and determine activation energies for chemical reactions, the relative amounts and functioning of the variety of enzymes within a cell ultimately determine which reactions will proceed and at which rates. This determination is tightly controlled. In certain cellular environments, enzyme activity is partly controlled by environmental factors, like pH and temperature. There are other mechanisms through which cells control the activity of enzymes and determine the rates at which various biochemical reactions will occur.

Regulation of Enzymes by Molecules
Enzymes can be regulated in ways that either promote or reduce their activity. There are many different kinds of molecules that inhibit or promote enzyme function, and various mechanisms exist for doing so. In some cases of enzyme inhibition, for example, an inhibitor molecule is similar enough to a substrate that it can bind to the active site and simply block the substrate from binding. When this happens, the enzyme is inhibited through competitive inhibition , because an inhibitor molecule competes with the substrate for active site binding ( Figure 6.17 ). On the other hand, in noncompetitive inhibition , an inhibitor molecule binds to the enzyme in a location other than the active site, called an allosteric site, but still manages to prevent substrate binding to the active site. Some inhibitor molecules bind to enzymes in a location where their binding induces a conformational change that reduces the enzyme activity as it no longer effectively catalyzes the conversion of the substrate to product.
Some inhibitor molecules bind to enzymes in a location where their binding induces a conformational change that reduces the affinity of the enzyme for its substrate. This type of inhibition is called allosteric inhibition ( Figure 6.18 ). Most allosterically regulated enzymes are made up of more than one polypeptide, meaning that they have more than one protein subunit. When an allosteric inhibitor binds to an enzyme, all active sites on the protein subunits are changed slightly such that they bind their substrates with less efficiency. There are allosteric activators as well as inhibitors. Allosteric activators bind to locations on an enzyme away from the active site, inducing a conformational change that increases the affinity of the enzyme’s active site(s) for its substrate(s).
Everyday Connection
Drug discovery by looking for inhibitors of key enzymes in specific pathways.
Enzymes are key components of metabolic pathways. Understanding how enzymes work and how they can be regulated is a key principle behind the development of many of the pharmaceutical drugs ( Figure 6.19 ) on the market today. Biologists working in this field collaborate with other scientists, usually chemists, to design drugs.
Consider statins for example—which is the name given to the class of drugs that reduces cholesterol levels. These compounds are essentially inhibitors of the enzyme HMG-CoA reductase. HMG-CoA reductase is the enzyme that synthesizes cholesterol from lipids in the body. By inhibiting this enzyme, the levels of cholesterol synthesized in the body can be reduced. Similarly, acetaminophen is an inhibitor of the enzyme cyclooxygenase. While it is effective in providing relief from fever and inflammation (pain), its mechanism of action is still not completely understood.
How are drugs developed? One of the first challenges in drug development is identifying the specific molecule that the drug is intended to target. In the case of statins, HMG-CoA reductase is the drug target. Drug targets are identified through painstaking research in the laboratory. Identifying the target alone is not sufficient; scientists also need to know how the target acts inside the cell and which reactions go awry in the case of disease. Once the target and the pathway are identified, then the actual process of drug design begins. During this stage, chemists and biologists work together to design and synthesize molecules that can either block or activate a particular reaction. However, this is only the beginning: both if and when a drug prototype is successful in performing its function, then it must undergo many tests from in vitro experiments to clinical trials before it can get FDA approval to be on the market.
- a drug that increases HMG-CoA reductase levels
- a drug that reduces cyclooxygenase levels
- a drug that reduces lipid levels in the body
- a drug that blocks the action of acetaminophen
Many enzymes don’t work optimally, or even at all, unless bound to other specific non-protein helper molecules, either temporarily through ionic or hydrogen bonds or permanently through stronger covalent bonds. Two types of helper molecules are cofactors and coenzymes . Binding to these molecules promotes optimal conformation and function for their respective enzymes. Cofactors are inorganic ions such as iron (Fe++) and magnesium (Mg++). One example of an enzyme that requires a metal ion as a cofactor is the enzyme that builds DNA molecules, DNA polymerase, which requires a bound zinc ion (Zn++) to function. Coenzymes are organic helper molecules, with a basic atomic structure made up of carbon and hydrogen, which are required for enzyme action. The most common sources of coenzymes are dietary vitamins ( Figure 6.20 ). Some vitamins are precursors to coenzymes and others act directly as coenzymes. Vitamin C is a coenzyme for multiple enzymes that take part in building the important connective tissue component, collagen. An important step in the breakdown of glucose to yield energy is catalysis by a multi-enzyme complex called pyruvate dehydrogenase. Pyruvate dehydrogenase is a complex of several enzymes that actually requires one cofactor (a magnesium ion) and five different organic coenzymes to catalyze its specific chemical reaction. Therefore, enzyme function is, in part, regulated by an abundance of various cofactors and coenzymes, which are supplied primarily by the diets of most organisms.
Enzyme Compartmentalization
In eukaryotic cells, molecules such as enzymes are usually compartmentalized into different organelles. This allows for yet another level of regulation of enzyme activity. Enzymes required only for certain cellular processes can be housed separately along with their substrates, allowing for more efficient chemical reactions. Examples of this sort of enzyme regulation based on location and proximity include the enzymes involved in the latter stages of cellular respiration, which take place exclusively in the mitochondria, and the enzymes involved in the digestion of cellular debris and foreign materials, located within lysosomes.
Feedback Inhibition in Metabolic Pathways
Molecules can regulate enzyme function in many ways. A major question remains, however: What are these molecules and where do they come from? Some are cofactors and coenzymes, ions, and organic molecules, as you’ve learned. What other molecules in the cell provide enzymatic regulation, such as allosteric modulation, and competitive and noncompetitive inhibition? The answer is that a wide variety of molecules can perform these roles. Some of these molecules include pharmaceutical and non-pharmaceutical drugs, toxins, and poisons from the environment. Perhaps the most relevant sources of enzyme regulatory molecules, with respect to cellular metabolism, are the products of the cellular metabolic reactions themselves. In a most efficient and elegant way, cells have evolved to use the products of their own reactions for feedback inhibition of enzyme activity. Feedback inhibition involves the use of a reaction product to regulate its own further production ( Figure 6.21 ). The cell responds to the abundance of specific products by slowing down production during anabolic or catabolic reactions. Such reaction products may inhibit the enzymes that catalyzed their production through the mechanisms described above.
The production of both amino acids and nucleotides is controlled through feedback inhibition. Additionally, ATP is an allosteric regulator of some of the enzymes involved in the catabolic breakdown of sugar, the process that produces ATP. In this way, when ATP is abundant, the cell can prevent its further production. Remember that ATP is an unstable molecule that can spontaneously dissociate into ADP. If too much ATP were present in a cell, much of it would go to waste. On the other hand, ADP serves as a positive allosteric regulator (an allosteric activator) for some of the same enzymes that are inhibited by ATP. Thus, when relative levels of ADP are high compared to ATP, the cell is triggered to produce more ATP through the catabolism of sugar.
Ask students which inhibition is more effective at slowing or limiting the reaction? Relate this to the examples available and discuss why these would be used in specific instances.
Have the class research antimicrobial treatments that are based on enzyme inhibition, not on the administration of traditional antibiotics.
Enzymes are not changed by the chemicals they facilitate; therefore, they can be used repeatedly. Yet, how do you keep them from catalyzing reactions when you do not need or want them to react anymore? If enzymes could not be controlled, the reactions would continue until the substrates were depleted, which is not a good situation for a living organism. Competitive and noncompetitive inhibition explains the control of enzyme activity. Research several examples of both in living organisms and explain why they are necessary. Amino acid production is one useful example. Amino acids are required for protein production, but too high a level of any amino acid is toxic, so the pathways must be controlled. Use the feedback inhibition of several pathways as examples.
As an Amazon Associate we earn from qualifying purchases.
This book may not be used in the training of large language models or otherwise be ingested into large language models or generative AI offerings without OpenStax's permission.
Want to cite, share, or modify this book? This book uses the Creative Commons Attribution License and you must attribute OpenStax.
Access for free at https://openstax.org/books/biology-ap-courses/pages/1-introduction
- Authors: Julianne Zedalis, John Eggebrecht
- Publisher/website: OpenStax
- Book title: Biology for AP® Courses
- Publication date: Mar 8, 2018
- Location: Houston, Texas
- Book URL: https://openstax.org/books/biology-ap-courses/pages/1-introduction
- Section URL: https://openstax.org/books/biology-ap-courses/pages/6-5-enzymes
© Jan 8, 2024 OpenStax. Textbook content produced by OpenStax is licensed under a Creative Commons Attribution License . The OpenStax name, OpenStax logo, OpenStax book covers, OpenStax CNX name, and OpenStax CNX logo are not subject to the Creative Commons license and may not be reproduced without the prior and express written consent of Rice University.
Find what you need to study
AP Biology Free Response Questions (FRQ) – Past Prompts
34 min read • december 29, 2020
Dylan Black
We’ve compiled a list of a bunch of the AP Biology past prompts! The AP Bio FRQs are 60% of the exam including 2 long questions and 4 short questions. It’s important that you understand the rubrics and question styles going into the exam. Use this list to practice!
By practicing with previously released free-response questions (FRQs), you’ll build critical-thinking and analytical skills that will prepare you for the exam. These past prompts have been designed to help you connect concepts and ideas to each other while applying your knowledge to real-life scenarios. You’ll also learn how to tackle the exam in a better format, and you won’t be surprised come test day with certain questions.
All recredit to College Board.
👉 AP Bio 2019 FRQs
Long FRQ #1
Gene expression and regulation, ecology (gene expression and symbiosis).
Auxins are plant hormones that coordinate several aspects of root growth and development. Indole-3-acetic acid (IAA) is an auxin that is usually synthesized from the amino acid tryptophan (Figure 1). Gene Trp-T encodes an enzyme that converts tryptophan to indole-3-pyruvic acid (I3PA), which is then converted to IAA by an enzyme encoded by the gene YUC .
Circle ONE arrow that represents transcription on the template pathway. Identify the molecule that would be absent if enzyme YUC is nonfunctional.
Predict how the deletion of one base pair in the fourth codon of the coding region of gene Trp-T would most likely affect the production of IAA. Justify your prediction.
Explain one feedback mechanism by which a cell could prevent production of too much IAA without limiting I3PA production
Rhizobacteria are a group of bacteria that live in nodules on plant roots. Rhizobacteria can produce IAA and convert atmospheric nitrogen into forms that can be used by plants. Plants release carbon-containing molecules into the nodules. Based on this information, identify the most likely ecological relationship between plants and rhizobacteria. Describe ONE advantage to the bacteria of producing IAA.
A researcher removed a plant nodule and identified several “cheater” rhizobacteria that do not produce IAA or fix nitrogen. Describe the evolutionary advantage of being a bacterial cheater in a population composed predominantly of noncheater bacteria. Plants can adjust the amount of carbon-containing molecules released into nodules in response to the amount of nitrogen fixed in the nodule. Predict the change in the bacterial population that would cause the plant to reduce the amount of carbon-containing molecules provided to the nodule.
Long FRQ #2
Units 2 and 8 (competition and osmoregulation).
A student studying two different aquatic, plant-eating, unicellular protist species (species A and B) designed an experiment to investigate the ecological relationship between the two species (Table 1).
In treatment group I, the student placed 10 individuals of species A into a container with liquid growth medium and 10 individuals of species B into a separate container with an equal amount of the same liquid growth medium. In treatment group II, the student placed 5 individuals of each species into a single container with the liquid growth medium. The student then maintained the containers under the same environmental conditions and recorded the number of individuals in each population at various time points. The results are shown in Table 2.
The growth curves for species B in group I and for species A in group II (shaded columns) have been plotted on the template. Use the template to complete an appropriately labeled line graph to illustrate the growth of species A in treatment group I and species B in treatment group II (unshaded columns).
As shown in the table, the student established treatment group II with 5 individuals of each species. Provide reasoning for the reduced initial population sizes.
The student claims that species A and B compete for the same food source. Provide TWO pieces of evidence from the data that support the student’s claim.
Predict TWO factors that will most likely limit the population growth of species A in treatment group I.
Many protists contain an organelle called a contractile vacuole that pumps water out of the cell. The student repeated the experiment using a growth medium with a lower solute concentration. Predict how the activity of the contractile vacuole will change under the new experimental conditions. Justify your prediction.
Short FRQ #1
Cellular energetics, heredity (cellular respiration and sex linked inheritance).
The pyruvate dehydrogenase complex (PDC) catalyzes the conversion of pyruvate to acetyl-CoA, a substrate for the Krebs (citric acid) cycle. The rate of pyruvate conversion is greatly reduced in individuals with PDC deficiency, a rare disorder.
Identify the cellular location where PDC is most active.
Make a claim about how PDC deficiency affects the amount of NADH produced by glycolysis AND the amount of NADH produced by the Krebs (citric acid) cycle in a cell. Provide reasoning to support your claims based on the position of the PDC-catalyzed reaction in the sequence of the cellular respiration pathway.
PDC deficiency is caused by mutations in the PDHA1 gene, which is located on the X chromosome. A male with PDC deficiency and a homozygous female with no family history of PDC deficiency have a male offspring. Calculate the probability that the male offspring will have PDC deficiency.
Short FRQ #2
Cell communication and cell cycle (cell signaling).
Acetylcholine is a neurotransmitter that can activate an action potential in a postsynaptic neuron (Figures 1 and 2). A researcher is investigating the effect of a particular neurotoxin that causes the amount of acetylcholine released from presynaptic neurons to increase.
Describe the immediate effect of the neurotoxin on the number of action potentials in a postsynaptic neuron. Predict whether the maximum membrane potential of the postsynaptic neuron will increase, decrease, or stay the same.
The researcher proposes two models, A and B, for using acetylcholinesterase (AChE), an enzyme that degrades acetylcholine, to prevent the effect of the neurotoxin. In model A, AChE is added to the synapse. In model B, AChE is added to the cytoplasm of the postsynaptic cell. Predict the effectiveness of EACH proposed model. Provide reasoning to support your predictions.
Short FRQ #3
Natural selection (cladograms and evolutionary relationships).
A researcher studying the evolutionary relationship among five primate species obtained data from a sequence of mitochondrial DNA (mtDNA) from a representative individual of each species. The researcher then calculated the percent divergence in the sequences between each pair of primate species (Table 1).
Based on fossil data, the researcher estimates that humans and their most closely related species in the data set diverged approximately seven million years ago. Using these data, calculate the rate of mtDNA percent divergence per million years between humans and their most closely related species in the data set. Round your answer to two decimal places.
Using the data in the table, construct a cladogram on the template provided. Provide reasoning for the placement of gibbons as the outgroup on the cladogram.
On the cladogram, draw a circle around all of the species that are descended from the species indicated by the node within the square.
Short FRQ #4
Cell communication and cell cycle (gene expression).
The yeast Saccharomyces cerevisiae is a single-celled organism. Amino acid synthesis in yeast cells occurs through metabolic pathways, and enzymes in the synthesis pathways are encoded by different genes. The synthesis of a particular amino acid can be prevented by mutation of a gene encoding an enzyme in the required pathway. A researcher conducted an experiment to determine the ability of yeast to grow on media that differ in amino acid content. Yeast can grow as both haploid and diploid cells. The researcher tested two different haploid yeast strains (Mutant 1 and Mutant 2), each of which has a single recessive mutation, and a haploid wild-type strain. The resulting data are shown in Table 1.
Identify the role of treatment I in the experiment.
Provide reasoning to explain how Mutant 1 can grow on treatment I medium but cannot grow on treatment III medium.
Yeast mate by fusing two haploid cells to make a diploid cell. In a second experiment, the researcher mates the Mutant 1 and Mutant 2 haploid strains to produce diploid cells. Using the table provided, predict whether the diploid cells will grow on each of the four media. Use a plus sign (+) to indicate growth and a minus sign (−) to indicate no growth.
Short FRQ #5
A researcher is studying patterns of gene expression in mice. The researcher collected samples from six different tissues in a healthy mouse and measured the amount of mRNA from six genes. The data are shown in Figure 1.
Based on the data provided, identify the gene that is most likely to encode a protein that is an essential component of glycolysis. Provide reasoning to support your identification.
The researcher observed that tissues with a high level of gene H mRNA did not always have gene H protein. Provide reasoning to explain how tissues with high gene H mRNA levels can have no gene H protein.
Short FRQ #6
Cell structure and function (cellular transport).
The petal color of the Mexican morning glory (Ipomoea tricolor ) changes from red to blue, and the petal cells swell during flower opening. The pigment heavenly blue anthocyanin is found in the vacuole of petal cells. Petal color is determined by the pH of the vacuole. A model of a morning glory petal cell before and after flower opening is shown in Table 1.
Identify the cellular component in the model that is responsible for the increase in the pH of the vacuole during flower opening AND describe the component’s role in changing the pH of the vacuole.
A researcher claims that the activation of the K+/H+ transport protein causes the vacuole to swell with water. Provide reasoning to support the researcher’s claim.
👉 AP Bio 2018 FRQs
Polar bears are highly adapted for life in cold climates around the North Pole. Brown bears, black bears, and pandas are found in warmer environments. Researchers collected complete mitochondrial DNA sequences from several populations of bears and constructed a phylogenetic tree to represent their evolutionary relatedness (Figure 1). A researcher studying adaptation in bears sequenced the nuclear gene encoding a lysosomal trafficking protein (LYST) in polar bears, brown bears, black bears, and panda bears. There are seven inferred amino acid substitutions that are found only in polar bears. Mutations that cause similar substitutions in the human LYST protein are associated with Chediak-Higashi syndrome, an autosomal recessive condition in which pigment is absent from the hair and eyes. The researcher used the inferred amino acid sequences to build the distance matrix shown in Table 1.
Use the phylogenetic tree in Figure 1 to estimate the age in hundreds of thousands of years of the most recent common ancestor of all brown bears. Identify the population of brown bears to which polar bears are most closely related based on the mitochondrial DNA sequence comparison. Identify two populations whose positions could be switched without affecting the relationships illustrated in the phylogenetic tree.
Construct a cladogram on the template to represent a model of the evolutionary relatedness among the bear species based on the differences in LYST protein sequences (Table 1). Circle the position on the cladogram that represents the out-group.
A student claims that mitochondrial DNA sequence comparisons provide a more accurate phylogeny of bear species than do LYST protein sequence comparisons. Provide ONE piece of reasoning to support the student’s claim.
A researcher genetically engineers a mouse strain by deleting the mouse lyst gene and replacing it with the polar bear lyst gene. Predict the most likely difference in phenotype of the transgenic mouse strain compared to the wild-type mouse strain. Justify your prediction.
Describe how the mutation in the lyst gene became common in the polar bear population. If the lyst gene were the only determinant of fur color, predict the percent of white offspring produced by a mating between a polar bear and a brown bear.
Cell Communication and Cell Cycle (Gene Regulation)
Some pathogenic bacteria enter cells, replicate, and spread to other cells, causing illness in the host organism. Host cells respond to these infections in a number of ways, one of which involves activating particular enzymatic pathways (Figure 1). Cells normally produce a steady supply of inactive caspase-1 protein. In response to intracellular pathogens, the inactive caspase-1 is cleaved and forms an active caspase-1 (step 1). Active caspase-1 can cleave two other proteins. When caspase-1 cleaves an inactive interleukin (step 2), the active portion of the interleukin is released from the cell. An interleukin is a signaling molecule that can activate the immune response. When caspase-1 cleaves gasdermin (step 3), the N-terminal portions of several gasdermin proteins associate in the cell membrane to form large, nonspecific pores. Researchers created the model in Figure 1 using data from cell fractionation studies. In the experiments, various parts of the cell were separated into fractions by mechanical and chemical methods. Specific proteins known to be located in different parts of the cell were used as markers to determine the location of other proteins. The table below shows the presence of known proteins in specific cellular fractions.
Describe the effect of inhibiting step 3 on the formation of pores AND on the release of interleukin from the cell.
Make a claim about how cleaving inactive caspase-1 results in activation of caspase-1. A student claims that preinfection production of inactive precursors shortens the response time of a cell to a bacterial infection. Provide ONE reason to support the student’s claim.
A student claims that the NF-kB protein is located in the cytoplasm until the protein is needed for transcription. Justify the student’s claim with evidence. Identify TWO fractions where N-terminal gasdermin would be found in cells infected with pathogenic bacteria.
Describe the most likely effect of gasdermin pore formation on water balance in the cell in a hypotonic environment.
Explain how gasdermin pore formation AND interleukin release contribute to an organism’s defense against a bacterial pathogen.
Seagrasses are aquatic plants that reproduce sexually. Male seagrass flowers produce sticky pollen that is carried by circulating water to female flowers, resulting in fertilization. A researcher claims that mobile aquatic invertebrates can also transfer pollen from male to female flowers in the absence of circulating water. To investigate this claim, the researcher set up aquariums to model the possible interactions between the invertebrates and seagrasses.
Use the symbols below and the template aquariums to demonstrate the experimental design for testing the researcher’s claim that mobile aquatic invertebrates can pollinate seagrass in the absence of circulating water. Draw the appropriate symbols in the negative control aquarium AND the experimental aquarium. Do not use any symbol more than once in the same aquarium.
Identify the dependent variable in the experiment. Predict the experimental results that would support the researcher’s claim that mobile aquatic invertebrates can also transfer pollen from male to female flowers in the absence of circulating water.
Cell Structure and Function (Cell Transport and Experimental Design)
The common bedbug ( Cimex lectularius ) is a species of insect that is becoming increasingly resistant to insecticides. Bedbugs possess several genes suspected of contributing to the resistance, including P450 , Abc8 , and Cps . To investigate the role of these genes in insecticide resistance, researchers deleted one or more of these genes in different strains of bedbugs, as indicated in Figure 1, and treated the strains with the insecticide beta-cyfluthrin. Each strain was genetically identical except for the deleted gene(s) and was equally fit in the absence of beta-cyfluthrin. The percent survival of each strain following beta-cyfluthrin treatment is shown in Figure 1.
Identify the control strain in the experiment. Use the means and confidence intervals in Figure 1 to justify the claim that Abc8 is effective at providing resistance to beta-cyfluthrin.
P450 encodes an enzyme that detoxifies insecticides. Abc8 encodes a transporter protein that pumps insecticides out of cells. Cps encodes an external structural protein located in the exoskeleton that greatly reduces the absorption of insecticides. Based on this information and the data in Figure 1, explain how a deletion of both P450 and Abc8 results in lower survival in bedbugs compared with a deletion of Cps only.
Ecology (Symbiotic Relationships)
Some birds, including great spotted cuckoos, lay their eggs in the nests of other birds, such as reed warblers. The warbler parents raise the unrelated chicks and provide them with food that would otherwise be given to their biological offspring. A researcher conducted an investigation to determine the type of relationship between warblers and cuckoos in an environment without predators. The researcher found that nests containing only warblers were more likely to be successful than nests containing warblers and cuckoos (data not shown). A successful nest is defined as a nest where at least one chick becomes an adult warbler. In some geographic areas, several species of nest predators are present. Researchers have found that cuckoo chicks, while in the nest, produce a smelly substance that deters nest predators. The substance does not remain in the nest if cuckoo chicks are removed. Figure 1 shows the probability that nests containing only warblers or containing both warblers and cuckoos will be successful in an environment with predators. In a follow-up experiment, the researchers added cuckoos to a nest that contained only warblers (group 1) and removed cuckoos from a nest containing warblers and cuckoos (group 2).
Describe the symbiotic relationship that exists between the cuckoo and warbler in an environment without predators.
On the template provided, draw bars in the appropriate locations to predict the relative probability of success for the nest in the presence of predators where:
the cuckoos were added to the nest containing only warblers (group 1)
the cuckoos were removed from the nest containing warblers and cuckoos (group 2)
Identify the symbiotic relationship that exists between the cuckoo and the warbler in the presence of predators.
Cystic fibrosis is a genetic condition that is associated with defects in the CFTR protein. The CFTR protein is a gated ion channel that requires ATP binding in order to allow chloride ions (Cl−) to diffuse across the membrane.
In the provided model of a cell, draw arrows to describe the pathway for production of a normal CFTR protein from gene expression to final cellular location.
Identify the most likely cellular location of the ribosomes that synthesize CFTR protein.
Identify the most likely cellular location of a mutant CFTR protein that has an amino acid substitution in the ATP-binding site.
Heredity (Sex Linked Heredity)
In the tongue sole fish (Cynoglossus semilaevis), sex is determined by a combination of genetics and environmental temperature. Genetically male fish have two Z chromosomes (ZZ), and genetically female fish have one Z chromosome and one W chromosome (ZW). When fish are raised at 22℃, ZZ fish develop into phenotypic males and ZW fish develop into phenotypic females. However, when fish are raised at 28℃, the Z chromosome is modified (denoted as Z*). Z*W individuals develop as phenotypic males that are fertile and can pass on the Z* chromosome to their offspring even when the offspring are raised at 22℃. A cross between a ZW female and a Z*Z male is shown in the Punnett square below.
Predict the percent of phenotypic males among the F1 offspring of the cross shown in the Punnett square if the offspring are raised at 22℃.
At least one Z or Z* chromosome is necessary for survival of the fish. A researcher crossed two fish and observed a 2:1 ratio of males to females among the offspring. Based on the information, identify the genotype of the male parent in the cross. Describe ONE fitness cost to the female of mating with this particular male.
Cell Communication and Cell Cycle
Acetylcholine receptor (AChR) proteins are found at the synapse between neurons and skeletal muscle cells. Acetylcholine released from neurons binds to a specific site on the receptor proteins, which causes an ion channel in the receptors to open and allow sodium ions (Na+) to enter muscle cells. The resulting depolarization of muscle cells initiates muscle contractions. Another molecule, nicotine, can also bind to certain types of AChR proteins and activate the receptors.A researcher is investigating two different types of AChR proteins: type 1 and type 2. To determine which stimuli activate the receptors, the researcher exposes muscle cells expressing the different types of receptor proteins to stimuli and observes the results indicated in Table 1.
Describe the difference in the structure AND function between AChR type 1 and AChR type 2.
Acetylcholinesterase is an enzyme that breaks down acetylcholine in the synapse. Describe the effect of inhibiting acetylcholinesterase on the muscle cells with AChR type 2.
👉 AP Bio 2017 FRQs
Experimental Design and Cellular Energetics
In flowering plants, pollination is a process that leads to the fertilization of an egg and the production of seeds. Some flowers attract pollinators, such as bees, using visual and chemical cues. When a bee visits a flower, in addition to transferring pollen, the bee can take nectar from the flower and use it to make honey for the colony. Nectar contains sugar, but certain plants also produce caffeine in the nectar. Caffeine is a bitter-tasting compound that can be toxic to insects at high concentrations. To investigate the role of caffeine in nectar, a group of researchers studied the effect of 0.1 mM caffeine on bee behavior. The results of an experiment to test the effect of caffeine on bees’ memory of a nectar source are shown in Table 1.
On the axes provided, construct an appropriately labeled graph to illustrate the effect of caffeine on the probability of bees revisiting a nectar source (memory).
Based on the results, describe the effect of caffeine on each of the following:
Short-term (10 minute) memory of a nectar source
Long-term (24 hour) memory of a nectar source
Design an experiment using artificial flowers to investigate potential negative effects of increasing caffeine concentrations in nectar on the number of floral visits by bees. Identify the null hypothesis, an appropriate control treatment, and the predicted results that could be used to reject the null hypothesis.
Researchers found that nectar with caffeine tends to have a lower sugar content than nectar without caffeine. Plants use less energy to produce the caffeine in nectar than they do to produce the sugar in nectar. Propose ONE benefit to plants that produce nectar with caffeine and a lower sugar content. Propose ONE cost to bees that visit the flowers of plants that produce nectar with caffeine and a lower sugar content.
Ecology (Ecological Succession)
Fires frequently occur in some ecosystems and can destroy all above-ground vegetation. Many species of plants in these ecosystems respond to compounds in smoke that regulate seed germination after a major fire. Karrikins (KAR) and trimethylbutenolides (TMB) are water-soluble compounds found in smoke that are deposited in the soil as a result of a fire. KAR and TMB bind to receptor proteins in a seed. In a study on the effects of smoke on seeds, researchers recorded the timing and percent of seed germination in the presence of various combinations of KAR and TMB. The results are shown in Figure 1. In a second investigation into the effect of available water on seed germination after a fire, researchers treated seeds with KAR or TMB. The treated seeds were then divided into two treatment groups. One group received a water rinse and the other group received no water rinse. The seeds were then incubated along with a group of control seeds that were not treated. The results are shown in the table.
The researchers made the following claims about the effect of KAR and the effect of TMB on seed germination relative to the control treatment.
KAR alone affects the timing of seed germination.
KAR alone affects the percentage of seeds that germinate.
TMB alone affects the timing of seed germination.
TMB alone affects the percentage of seeds that germinate.
Provide support using data from Figure 1 for each of the researchers’ claims.
Make a claim about the effect of rinsing on the binding of KAR to the receptor in the seed and about the effect of rinsing on the binding of TMB to the receptor in the seed. Identify the appropriate treatment groups and results from the table that, when compared with the controls, provide support for each claim.
There is intense competition by plants to successfully colonize areas that have been recently cleared by a fire. Describe ONE advantage of KAR regulation and ONE advantage of TMB regulation to plants that live in an ecosystem with regular fires.
Cell Communication and Cell Cycle (Gene Regulation and Expression)
Gibberellin is the primary plant hormone that promotes stem elongation. GA 3-beta-hydroxylase (GA3H) is the enzyme that catalyzes the reaction that converts a precursor of gibberellin to the active form of gibberellin. A mutation in the GA3H gene results in a short plant phenotype. When a pure-breeding tall plant is crossed with a pure-breeding short plant, all offspring in the F1 generation are tall. When the F1 plants are crossed with each other, 75 percent of the plants in the F2 generation are tall and 25 percent of the plants are short.
The wild-type allele encodes a GA3H enzyme with alanine (Ala), a nonpolar amino acid, at position 229. The mutant allele encodes a GA3H enzyme with threonine (Thr), a polar amino acid, at position 229. Describe the effect of the mutation on the enzyme and provide reasoning to support how this mutation results in a short plant phenotype in homozygous recessive plants.
Using the codon chart provided, predict the change in the codon sequence that resulted in the substitution of alanine for threonine at amino acid position 229.
Describe how individuals with one (heterozygous) or two (homozygous) copies of the wild-type GA3H allele can have the same phenotype.
Ecology (Energy Diagrams)
The table above shows how much each organism in an aquatic ecosystem relies on various food sources. The rows represent the organisms in the ecosystem, and the columns represent the food source. The percentages indicate the proportional dietary composition of each organism. High percentages indicate strong dependence of an organism on a food source.
Based on the food sources indicated in the data table, construct a food web in the template below. Write the organism names on the appropriate lines AND draw the arrows necessary to indicate the energy flow between organisms in the ecosystem.
In an effort to control the number of midges, an area within the ecosystem was sprayed with the fungus Metarhizium anisopliae, which significantly decreased the midge population. Based on the data in the table, predict whether the spraying of the fungus will have the greatest short-term impact on the population of the stoneflies, the caddisflies, or the hellgrammites. Justify your prediction.
Cellular Energetics
Microcystis aeruginosis is a freshwater photosynthetic cyanobacterium. When temperatures increase and nutrients are readily available in its pond habitat, M. aeruginosis undergoes rapid cell division and forms an extremely large, visible mass of cells called an algal bloom. M. aeruginosis has a short life span and is decomposed by aerobic bacteria and fungi. Identify the metabolic pathway and the organism that is primarily responsible for the change in oxygen level in the pond between times I and II AND between times III and IV.
Cell Communication and Cell Cycle (DNA Structure and Function)
A comet assay is a technique used to determine the amount of double-strand breaks in DNA (DNA damage) in cells. The nucleus of an individual cell is placed on a microscope slide coated with an agarose gel. An electric current is applied to the gel that causes DNA to move (electrophoresis), and the DNA is stained with a fluorescent dye. When viewed using a microscope, undamaged DNA from the nucleus appears as a round shape (the head), and the fragments of damaged DNA extend out from the head (the tail). The length of the tail corresponds to the amount of the damage in the DNA (see Figure 1).
To explain the movement of DNA fragments in the comet assay, identify one property of DNA and provide reasoning to support how the property contributes to the movement during the comet assay technique.
In a different experiment, cells are treated with a chemical mutagen that causes only nucleotide substitutions in DNA. Predict the likely results of a comet assay for this treatment.
Cellular Energetics (Anaerobic Respiration)
Many species of bacteria grow in the mouths of animals and can form biofilms on teeth (plaque). Within plaque, the outer layers contain high levels of oxygen and the layers closest to the tooth contain low levels of oxygen. The surface of the tooth is covered in a hard layer of enamel, which can be dissolved under acidic conditions. When the enamel breaks down, the bacteria in plaque can extract nutrients from the tooth and cause cavities.Certain types of bacteria (e.g., Streptococcus mutans) thrive in the innermost anaerobic layers of the plaque and are associated with cavities. Other types of bacteria (Streptococcus sanguinis) compete with S. mutans but are unable to thrive in acidic environments.
Identify the biochemical pathway S. mutans uses for metabolizing sugar and describe how the pathway contributes to the low pH in the inner layers of plaque.
Normal tooth brushing effectively removes much of the plaque from the flat surfaces of teeth but cannot reach the surfaces between teeth. Many commercial toothpastes contain alkaline components, which raise the pH of the mouth. Predict how the population sizes of S. mutans AND S. sanguinis in the bacterial community in the plaque between the teeth are likely to change when these toothpastes are used.
Cell Structure and Function Cell Transport
Estrogens are small hydrophobic lipid hormones that promote cell division and the development of reproductive structures in mammals. Estrogens passively diffuse across the plasma membrane and bind to their receptor proteins in the cytoplasm of target cells.
Describe ONE characteristic of the plasma membrane that allows estrogens to passively cross the membrane.
In a laboratory experiment, a researcher generates antibodies that bind to purified estrogen receptors extracted from cells. The researcher uses the antibodies in an attempt to treat estrogen-dependent cancers but finds that the treatment is ineffective. Explain the ineffectiveness of the antibodies for treating estrogen-dependent cancers.
👉 AP Bio 2016 FRQs
Cell Communication and Cell Cycle (Gene Regulation and Experimental Design)
Leucine aminopeptidases (LAPs) are found in all living organisms and have been associated with the response of the marine mussel, Mytilus edulis , to changes in salinity. LAPs are enzymes that remove N-terminal amino acids from proteins and release the free amino acids into the cytosol. To investigate the evolution of LAPs in wild populations of M. edulis , researchers sampled adult mussels from several different locations along a part of the northeast coast of the United States, as shown in Figure 1. The researchers then determined the percent of individuals possessing a particular lap allele, lap94 , in mussels from each sample site (table 1).
On the axes provided, construct an appropriately labeled bar graph to illustrate the observed frequencies of the lap 94 allele in the study populations.
Based on the data, describe the most likely effect of salinity on the frequency of the lap 94 allele in the marine mussel populations in Long Island Sound. Predict the likely lap 94 allele frequency at a sampling site between site 1 and site 2 in Long Island Sound.
Describe the most likely effect of LAP94 activity on the osmolarity of the cytosol. Describe the function of LAP94 in maintaining water balance in the mussels living in the Atlantic Ocean.
Marine mussel larvae are evenly dispersed throughout the study area by water movement. As larvae mature, they attach to the rocks in the water. Explain the differences in lap94 allele frequency among adult mussel populations at the sample sites despite the dispersal of larvae throughout the entire study area. Predict the likely effect on distribution of mussels in Long Island Sound if the lap94 allele was found in all of the mussels in the population. Justify your prediction.
Cell Communication and Cell Cycle and Ecology (Gene Regulation, Ecology, and Experimental Design)
Bacteria can be cultured in media with a carefully controlled nutrient composition. The graph above shows the growth of a bacterial population in a medium with limiting amounts of two nutrients, I and II.
Estimate the maximum population density in cells/mL for the culture. Using the data, describe what prevents further growth of the bacterial population in the culture.
Using the data, calculate the growth rate in cells/mL×hour of the bacterial population between hours 2 and 4.
Identify the preferred nutrient source of the bacteria in the culture over the course of the experiment. Use the graph to justify your response. Propose ONE advantage of the nutrient preference for an individual bacterium.
Describe how nutrient I most likely regulates the genes for metabolism of nutrient I and the genes for metabolism of nutrient II. Provide TWO reasons that the population does not grow between hours 5 and 6.
The graph above illustrates the percent dry weight of different parts of a particular annual plant (plants that live less than one year) from early May to late August. The percent dry weight can be used to estimate the amount of energy a plant uses to produce its leaves, vegetative buds, stems, roots, and reproductive parts (seeds, receptacles, and flowers).
Identify the direct source of the energy used for plant growth during the first week of May, and identify the part of the plant that grew the most during the same period.
Based on the data on the graph, estimate the percent of the total energy that the plant has allocated to the growth of leaves on the first day of July.
Compared with perennials (plants that live more than two years), annual plants often allocate a much greater percentage of their total energy to growth of their reproductive parts in any given year. Propose ONE evolutionary advantage of the energy allocation strategy in annual plants compared with that in perennial plants.
The figure represents the process of expression of gene X in a eukaryotic cell.
The primary transcript in the figure is 15 kilobases (kb) long, but the mature mRNA is 7 kb in length. Describe the modification that most likely resulted in the 8 kb difference in length of the mature mRNA molecule. Identify in your response the location in the cell where the change occurs.
Predict the length of the mature gene X mRNA if the full-length gene is introduced and expressed in prokaryotic cells. Justify your prediction.
Ecology (Ecological Relationships)
The graph above shows the mass of plants from two different species over time. The plants grew while attached to each other. The plants were separated at the time indicated by the vertical line in the graph. Using template 1, graph the predicted shape of the plant-mass lines after separation of the two plants if the plants were in an obligate mutualistic relationship. On template 2, graph the predicted shape of the plant-mass lines if the species 2 plant was a parasite of the species 1 plant. Justify each of your predictions.
Living and dead organisms continuously shed DNA fragments, known as eDNA, into the environment. To detect eDNA fragments in the environment, the polymerase chain reaction (PCR) can be used to amplify specific eDNA fragments. eDNA fragments of different lengths persist in the environment for varying amounts of time before becoming undetectable (Figure 1). To investigate whether silver carp, an invasive fish, have moved from a nearby river system into Lake Michigan, researchers tested water samples for the presence of eDNA specific to silver carp (Figure 2).
Justify the use of eDNA sampling as an appropriate technique for detecting the presence of silver carp in an environment where many different species of fish are found. Propose ONE advantage of identifying long eDNA fragments as opposed to short fragments for detecting silver carp.
The researchers tested a large number of water samples from Lake Michigan and found eDNA specific to silver carp in a single sample in the lake, as indicated in Figure 2. The researchers concluded that the single positive sample was a false positive and that no silver carp had entered Lake Michigan. Provide reasoning other than human error to support the researchers’ claim.
In a certain species of plant, the diploid number of chromosomes is 4 (2n = 4). Flower color is controlled by a single gene in which the green allele ( G ) is dominant to the purple allele ( g ). Plant height is controlled by a different gene in which the dwarf allele ( D ) is dominant to the tall allele ( d ). Individuals of the parental ( P ) generation with the genotypes GGDD and ggdd were crossed to produce F1 progeny.
Construct a diagram below to depict the four possible normal products of meiosis that would be produced by the F1 progeny. Show the chromosomes and the allele(s) they carry. Assume the genes are located on different chromosomes and the gene for flower color is on chromosome 1.
Predict the possible phenotypes and their ratios in the offspring of a testcross between an F1 individual and a ggdd individual.
If the two genes were genetically linked, describe how the proportions of phenotypes of the resulting offspring would most likely differ from those of the testcross between an F1 individual and a ggdd individual.
Researchers conducted a study to investigate the effect of exercise on the release of prolactin into the blood. The researchers measured the concentration of prolactin in the blood of eight adult males before (T = 0 hour) and after one hour (T = 1 hour) of vigorous exercise. As a control, the researchers measured the concentration of blood prolactin in the same group of individuals at the same times of day one week later, but without having them exercise. The results are shown in Figure 1.
Justify the use of the without-exercise treatment as the control in the study design.
Using evidence from the specific treatments, determine whether prolactin release changes after exercise. Justify your answer.
👉 AP Bio 2015 FRQs
Gene Expression and Regulation, Ecology (Symbiosis, Genetic Expression)
Many species have circadian rhythms that exhibit an approximately 24-hour cycle. Circadian rhythms are controlled by both genetics and environmental conditions, including light. Researchers investigated the effect of light on mouse behavior by using a running wheel with a motion sensor to record activity on actograms, as shown in Figure 1. For the investigation, adult male mice were individually housed in cages in a soundproof room at 25°C. Each mouse was provided with adequate food, water, bedding material, and a running wheel. The mice were exposed to daily periods of 12 hours of light (L) and 12 hours of dark (D) (L12:D12) for 14 days, and their activity was continuously monitored. The activity data are shown in Figure 2. After 14 days in L12:D12, the mice were placed in continuous darkness (DD), and their activity on the running wheel was recorded as before. The activity data under DD conditions are shown in Figure 3.
The nervous system plays a role in coordinating the observed activity pattern of the mice in response to light-dark stimuli. Describe ONE role of each of the following anatomical structures in responding to lightdark stimuli.
A photoreceptor in the retina of the eye
A motor neuron
Based on an analysis of the data in Figure 2, describe the activity pattern of the mice during the light and dark periods of the L12:D12 cycle.
The researchers claim that the genetically controlled circadian rhythm in the mice does not follow a 24-hour cycle. Describe ONE difference between the daily pattern of activity under L12:D12 conditions (Figure 2) and under DD conditions (Figure 3), and use the data to support the researchers’ claim.
To investigate the claim that exposure to light overrides the genetically controlled circadian rhythm, the researchers plan to repeat the experiment with mutant mice lacking a gene that controls the circadian rhythm. Predict the observed activity pattern of the mutant mice under L12:D12 conditions and under DD conditions that would support the claim that light overrides the genetically controlled circadian rhythm.
In nature, mice are potential prey for some predatory birds that hunt during the day. Describe TWO features of a model that represents how the predator-prey relationship between the birds and the mice may have resulted in the evolution of the observed activity pattern of the mice.
Cellular Energetics (Cellular Respiration, ATP, Krebs Cycle, Glycolysis)
Cellular respiration includes the metabolic pathways of glycolysis, the Krebs cycle, and the electron transport chain, as represented in the figures. In cellular respiration, carbohydrates and other metabolites are oxidized, and the resulting energy-transfer reactions support the synthesis of ATP.
Using the information above, describe ONE contribution of each of the following in ATP synthesis.
Catabolism of glucose in glycolysis and pyruvate oxidation
Oxidation of intermediates in the Krebs cycle
Formation of a proton gradient by the electron transport chain
Use each of the following observations to justify the claim that glycolysis first occurred in a common ancestor of all living organisms.
Nearly all existing organisms perform glycolysis.
Glycolysis occurs under anaerobic conditions.
Glycolysis occurs only in the cytosol.
A researcher estimates that, in a certain organism, the complete metabolism of glucose produces 30 molecules of ATP for each molecule of glucose. The energy released from the total oxidation of glucose under standard conditions is 686 kcal/mol. The energy released from the hydrolysis of ATP to ADP and inorganic phosphate under standard conditions is 7.3 kcal/mol. Calculate the amount of energy available from the hydrolysis of 30 moles of ATP. Calculate the efficiency of total ATP production from 1 mole of glucose in the organism. Describe what happens to the excess energy that is released from the metabolism of glucose.
The enzymes of the Krebs cycle function in the cytosol of bacteria, but among eukaryotes the enzymes function mostly in the mitochondria. Pose a scientific question that connects the subcellular location of the enzymes in the Krebs cycle to the evolution of eukaryotes.
Natural Selection, Chemical Structure of Life (Amino Acids, Phylogenic Tree)
The amino acid sequence of cytochrome c was determined for five different species of vertebrates. The table below shows the number of differences in the sequences between each pair of species.
Using the data in the table, create a phylogenetic tree on the template provided to reflect the evolutionary relationships of the organisms. Provide reasoning for the placement on the tree of the species that is least related to the others.
Identify whether morphological data or amino acid sequence data are more likely to accurately represent the true evolutionary relationships among the species, and provide reasoning for your answer.
Cell Communication and Cell Cycle (Mitosis, Meiosis)
Both mitosis and meiosis are forms of cell division that produce daughter cells containing genetic information from the parent cell.
Describe TWO events that are common to both mitosis and meiosis that ensure the resulting daughter cells inherit the appropriate number of chromosomes.
The genetic composition of daughter cells produced by mitosis differs from that of the daughter cells produced by meiosis. Describe TWO features of the cell division processes that lead to these differences.
Cellular Energetics (Photosynthesis)
Phototropism in plants is a response in which a plant shoot grows toward a light source. The results of five different experimental treatments from classic investigations of phototropism are shown above.
Give support for the claim that the cells located in the tip of the plant shoot detect the light by comparing the results from treatment group I with the results from treatment group II and treatment group III.
In treatment groups IV and V, the tips of the plants are removed and placed back onto the shoot on either a permeable or impermeable barrier. Using the results from treatment groups IV and V, describe TWO additional characteristics of the phototropism response.
Ecology (Population Dynamics)
In an attempt to rescue a small isolated population of snakes from decline, a few male snakes from several larger populations of the same species were introduced into the population in 1992. The snakes reproduce sexually, and there are abundant resources in the environment. The figure below shows the results of a study of the snake population both before and after the introduction of the outside males. In the study, the numbers of captured snakes indicate the overall population size.
Describe ONE characteristic of the original population that may have led to the population’s decline in size between 1989 and 1993.
Propose ONE reason that the introduction of the outside males rescued the snake population from decline.
Describe how the data support the statement that there are abundant resources in the environment.
Cell Communication and Cell Cycle (Cell signaling, transmissions)
Smell perception in mammals involves the interactions of airborne odorant molecules from the environment with receptor proteins on the olfactory neurons in the nasal cavity. The binding of odorant molecules to the receptor proteins triggers action potentials in the olfactory neurons and results in transmission of information to the brain. Mammalian genomes typically have approximately 1,000 functional odorant-receptor genes, each encoding a unique odorant receptor.
Describe how the signal is transmitted across the synapse from an activated olfactory sensory neuron to the interneuron that transmits the information to the brain.
Explain how the expression of a limited number of odorant receptor genes can lead to the perception of thousands of odors. Use the evidence about the number of odorant receptor genes to support your answer
Cell Communication and Cell Cycle (Cell Communication, Immune System)
An individual has lost the ability to activate B cells and mount a humoral immune response.
Propose ONE direct consequence of the loss of B-cell activity on the individual’s humoral immune response to the initial exposure to a bacterial pathogen.
Propose ONE direct consequence of the loss of B-cell activity on the speed of the individual’s humoral immune response to a second exposure to the bacterial pathogen.
Describe ONE characteristic of the individual’s immune response to the bacterial pathogen that is not affected by the loss of B cells.

Stay Connected
© 2024 Fiveable Inc. All rights reserved.
AP® and SAT® are trademarks registered by the College Board, which is not affiliated with, and does not endorse this website.

IMAGES
VIDEO
COMMENTS
Free-Response Questions. Download free-response questions from past exams along with scoring guidelines, sample responses from exam takers, and scoring distributions. If you are using assistive technology and need help accessing these PDFs in another format, contact Services for Students with Disabilities at 212-713-8333 or by email at ssd@info ...
**Updated on 1/31/24 to include the 2022-23 FRQ exams!**If you are looking for past AP Biology free-response questions (FRQs) that are organized by topic, then you have come to the right place. In this post, we have linked every freely available past FRQ there is from College Board and organized it into the following major topics of AP Biology. (Please note that we are not associated with ...
Clearly, enzymes are a necessary part of life! In this AP® Biology Crash Course Review, we went over the general structure of an enzyme and its activation site. We then reviewed what exactly enzymes do and how they do it. We then reviewed different types of activation and inhibition molecules. Finally, we wrapped up with an example of a real ...
The activator activates the enzyme by changing the shape of it to make the active site fit with the substrate. Feedback inhibition. prevents unnecessary accumulation of products by making the product used by the next step. the final product is an inhibitor of an earlier step. Study with Quizlet and memorize flashcards containing terms like What ...
Enzyme structure/function Describe experiment State hypothesis. OLD FRQ's prior to 1995 (1994) Enzymes are biological catalysts. a. Relate the chemical structure of an enzyme to its specificity and catalytic activity. b. Design a quantitative experiment to investigate the influence of pH or temperature on the activity of an enzyme.
5.1 The student can analyze data to identify patterns or relationships. Learning Objective. 4.17 The student is able to analyze data to identify how molecular interactions affect structure and function. The Science Practice Challenge Questions contain additional test questions for this section that will help you prepare for the AP exam.
The energy of the reactants is used to create the energy of the products, which is re-used for the energy of the new reactants. What is the role of enzymes in biological systems? The role of enzymes in biological systems are to reduce the amount of energy needed to begin a chemical reaction. What is the relationship between enzyme structure and ...
The flow of genetic information from DNA to protein in eukaryotic cells is called the central dogma of biology. (a) Explain the role of each of the following in protein synthesis in eukaryotic cells. RNA polymerase Spliceosomes (snRNPs) Codons Ribosomes tRNA (b) Cells regulate both protein synthesis and protein activity.
The enzymes of the Krebs cycle function in the cytosol of bacteria, but among eukaryotes the enzymes function mostly in the mitochondria. Pose a scientific question that connects the subcellular location of the enzymes in the Krebs cycle to the evolution of eukaryotes. ... AP Biology Free Response Questions (FRQ) - Past Prompts ...
The pyruvate dehydrogenase complex (PDC) catalyzes the conversion of pyruvate to acetyl-CoA, a substrate for the Krebs (citric acid) cycle. The rate of pyruvate conversion is greatly reduced in individuals with PDC deficiency, a rare disorder. (a) Identify the cellular location where PDC is most active.
FRQ #7: Enzymes are biological catalysts. Relate the chemical structure of an enzyme to its specificity and catalytic activity. Design a quantitative experiment to investigate the influence of pH or temperature on the activity of an enzyme. Describe what information concerning the structure of an enzyme could be inferred from your experiment.
AP® Biology 2022 Scoring Guidelines. Question 3: Scientific Investigation. 4 points. Fireflies emit light when the enzyme luciferase catalyzes a reaction in which its substrate, D-luciferin, reacts to form oxyluciferin and other products (Figure 1). In order to determine the optimal temperature for this enzyme, scientists added ATP to a ...
Inputs: glucose, takes 2 ATP to get going, 2 NAD+. Outputs: 2 pyruvate molecules, net 2 ATP, 2 NADH. Study with Quizlet and memorize flashcards containing terms like Describe the enzyme mediated process., Identify TWO environmental factors the impact enzymatic activity., Explain the effect of the two factors you identified. and more.
AP® Biology 2022 Scoring Guidelines . Question 1: Interpreting and Evaluating Experimental Results with Experimental Design 9 points . The binding of an extracellular ligand to a G protein-coupled receptor in the plasma membrane of a cell triggers intracellular signaling (Figure 1, A). After ligand binding, GTP replaces the GDP that is bound to
lowers the EA. what strcuture of the enzyme does denaturation change. denaturation changes the secondary and tertiary structures of the protein (enzyme) factors that modify enzyme function. pH, temperature, concentration of substrae/enzymes. Study with Quizlet and memorize flashcards containing terms like what is catabolism, what other ...