Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Published: 27 June 2019

The human stress response
- Georgina Russell 1 &
- Stafford Lightman ORCID: orcid.org/0000-0002-8546-9646 1
Nature Reviews Endocrinology volume 15 , pages 525–534 ( 2019 ) Cite this article
23k Accesses
396 Citations
833 Altmetric
Metrics details
- Adrenal cortex hormones
- Circadian rhythms
- Multihormonal system disorders
- Stress signalling
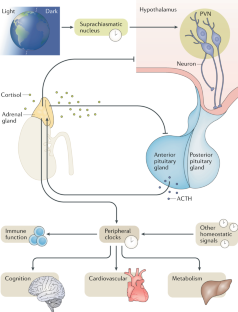
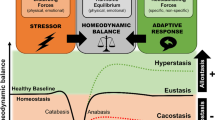
The human stress response has evolved to maintain homeostasis under conditions of real or perceived stress. This objective is achieved through autoregulatory neural and hormonal systems in close association with central and peripheral clocks. The hypothalamic–pituitary–adrenal axis is a key regulatory pathway in the maintenance of these homeostatic processes. The end product of this pathway — cortisol — is secreted in a pulsatile pattern, with changes in pulse amplitude creating a circadian pattern. During acute stress, cortisol levels rise and pulsatility is maintained. Although the initial rise in cortisol follows a large surge in adrenocorticotropic hormone levels, if long-term inflammatory stress occurs, adrenocorticotropic hormone levels return to near basal levels while cortisol levels remain raised as a result of increased adrenal sensitivity. In chronic stress, hypothalamic activation of the pituitary changes from corticotropin-releasing hormone-dominant to arginine vasopressin-dominant, and cortisol levels remain raised due at least in part to decreased cortisol metabolism. Acute elevations in cortisol levels are beneficial to promoting survival of the fittest as part of the fight-or-flight response. However, chronic exposure to stress results in reversal of the beneficial effects, with long-term cortisol exposure becoming maladaptive, which can lead to a broad range of problems including the metabolic syndrome, obesity, cancer, mental health disorders, cardiovascular disease and increased susceptibility to infections. Neuroimmunoendocrine modulation in disease states and glucocorticoid-based therapeutics are also discussed.
The hypothalamic–pituitary–adrenal (HPA) axis is a key system that synchronizes the stress response with circadian regulatory processes.
Regulation of the HPA axis is very dynamic with both ultradian and circadian oscillations.
Short-term and longer-term stress result in different regulatory mechanisms involving hypothalamic, pituitary and adrenal activity, as well as cortisol metabolism.
Chronic elevation and nonphysiological patterns of cortisol result in poor cognitive, metabolic and immune function.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
195,33 € per year
only 16,28 € per issue
Rent or buy this article
Prices vary by article type
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
The neuroendocrinology of stress: the stress-related continuum of chronic disease development
Agorastos Agorastos & George P. Chrousos

Systematic manipulations of the biological stress systems result in sex-specific compensatory stress responses and negative mood outcomes
Nida Ali, Jonas P. Nitschke, … Jens C. Pruessner
The cortisol switch between vulnerability and resilience
E. Ronald de Kloet & Marian Joëls
Szabo, S., Tache, Y. & Somogyi, A. The legacy of Hans Selye and the origins of stress research: a retrospective 75 years after his landmark brief “letter” to the editor of Nature . Stress 15 , 472–478 (2012).
CAS PubMed Google Scholar
Levine, S. Influence of psychological variables on the activity of the hypothalamic-pituitary-adrenal axis. Eur. J. Pharmacol. 405 , 149–160 (2000).
Brown, S. A. & Azzi, A. Peripheral circadian oscillators in mammals. Handb. Exp. Pharmacol. 2013 , 45–66 (2013).
Google Scholar
Roenneberg, T. & Merrow, M. The circadian clock and human health. Curr. Biol. 26 , R432–R443 (2016).
Bass, J. & Lazar, M. A. Circadian time signatures of fitness and disease. Science 354 , 994–999 (2016).
Turek, F. W. Circadian neural rhythms in mammals. Annu. Rev. Physiol. 47 , 49–64 (1985).
Skene, D. J. et al. Separation of circadian- and behavior-driven metabolite rhythms in humans provides a window on peripheral oscillators and metabolism. Proc. Natl Acad. Sci. USA 115 , 7825–7830 (2018).
Buhr, E. D. & Takahashi, J. S. Molecular components of the mammalian circadian clock. Handb. Exp. Pharmacol. 217 , 3–27 (2013).
CAS Google Scholar
Takahashi, J. S. Transcriptional architecture of the mammalian circadian clock. Nat. Rev. Genet. 18 , 164–179 (2017).
Baron, K. G. & Reid, K. J. Circadian misalignment and health. Int. Rev. Psychiatry 26 , 139–154 (2014).
PubMed PubMed Central Google Scholar
Potter, G. D. et al. Circadian rhythm and sleep disruption: causes, metabolic consequences, and countermeasures. Endocr. Rev. 37 , 584–608 (2016).
CAS PubMed PubMed Central Google Scholar
Zhang, R., Lahens, N. F., Ballance, H. I., Hughes, M. E. & Hogenesch, J. B. A circadian gene expression atlas in mammals: implications for biology and medicine. Proc. Natl Acad. Sci. USA 111 , 16219–16224 (2014).
Smarr, B. L. & Schirmer, A. E. 3.4 million real-world learning management system logins reveal the majority of students experience social jet lag correlated with decreased performance. Sci. Rep. 8 , 4793 (2018).
Gardner, M. et al. Dysregulation of the hypothalamic pituitary adrenal (HPA) axis and cognitive capability at older ages: individual participant meta-analysis of five cohorts. Sci. Rep. 9 , 4555 (2019).
Selye, H. Stress and the general adaptation syndrome. BMJ 1 , 1383–1392 (1950).
Russell, G. M. & Lightman, S. L. Can side effects of steroid treatments be minimized by the temporal aspects of delivery method? Expert Opin. Drug Saf. 13 , 1501–1513 (2014).
Sorrells, S. F. & Sapolsky, R. M. An inflammatory review of glucocorticoid actions in the CNS. Brain Behav. Immun. 21 , 259–272 (2007).
Busillo, J. M. & Cidlowski, J. A. The five Rs of glucocorticoid action during inflammation: ready, reinforce, repress, resolve, and restore. Trends Endocrinol. Metab. 24 , 109–119 (2013).
McEwen, B. S. et al. The role of adrenocorticoids as modulators of immune function in health and disease: neural, endocrine and immune interactions. Brain Res. Rev. 23 , 79–133 (1997).
Elenkov, I. J. & Chrousos, G. P. Stress system — organization, physiology and immunoregulation. Neuroimmunomodulation 13 , 257–267 (2006).
Brinkmann, V. & Kristofic, C. Regulation by corticosteroids of Th1 and Th2 cytokine production in human CD4+ effector T cells generated from CD45RO- and CD45RO+ subsets. J. Immunol. 155 , 3322–3328 (1995).
Wiegers, G. J. & Reul, J. M. Induction of cytokine receptors by glucocorticoids: functional and pathological significance. Trends Pharmacol. Sci. 19 , 317–321 (1998).
Abraham, I. M., Meerlo, P. & Luiten, P. G. Concentration dependent actions of glucocorticoids on neuronal viability and survival. Dose Response 4 , 38–54 (2006).
Plaschke, K., Muller, D. & Hoyer, S. Effect of adrenalectomy and corticosterone substitution on glucose and glycogen metabolism in rat brain. J. Neural Transm. 103 , 89–100 (1996).
Belanoff, J. K., Gross, K., Yager, A. & Schatzberg, A. F. Corticosteroids and cognition. J. Psychiatr. Res. 35 , 127–145 (2001).
Roozendaal, B. Stress and memory: opposing effects of glucocorticoids on memory consolidation and memory retrieval. Neurobiol. Learn. Mem. 78 , 578–595 (2002).
Brown, E. S. Effects of glucocorticoids on mood, memory, and the hippocampus. Treatment and preventive therapy. Ann. NY Acad. Sci. 1179 , 41–55 (2009).
de Kloet, E. R., Oitzl, M. S. & Joels, M. Stress and cognition: are corticosteroids good or bad guys? Trends Neurosci. 22 , 422–426 (1999).
PubMed Google Scholar
Decani, S., Federighi, V., Baruzzi, E., Sardella, A. & Lodi, G. Iatrogenic Cushing’s syndrome and topical steroid therapy: case series and review of the literature. J. Dermatolog. Treat. 25 , 495–500 (2014).
Kenna, H. A., Poon, A. W., de los Angeles, C. P. & Koran, L. M. Psychiatric complications of treatment with corticosteroids: review with case report. Psychiatry Clin. Neurosci. 65 , 549–560 (2011).
Buijs, R. M., Markman, M., Nunes-Cardoso, B., Hou, Y. X. & Shinn, S. Projections of the suprachiasmatic nucleus to stress-related areas in the rat hypothalamus: a light and electron microscopic study. J. Comp. Neurol. 335 , 42–54 (1993).
Watts, A. G. & Swanson, L. W. Efferent projections of the suprachiasmatic nucleus: II. Studies using retrograde transport of fluorescent dyes and simultaneous peptide immunohistochemistry in the rat. J. Comp. Neurol. 258 , 230–252 (1987).
Jacobson, L. Hypothalamic-pituitary-adrenocortical axis regulation. Endocrinol. Metab. Clin. North Am. 34 , 271–292 (2005).
Herman, J. P., Ostrander, M. M., Mueller, N. K. & Figueiredo, H. Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Prog. Neuropsychopharmacol. Biol. Psychiatry 29 , 1201–1213 (2005).
Dallman, M. F. et al. Corticosteroids and the control of function in the hypothalamo-pituitary-adrenal (HPA) axis. Ann. NY Acad. Sci. 746 , 22–31 (1994).
Jasper, M. S. & Engeland, W. C. Splanchnic neural activity modulates ultradian and circadian rhythms in adrenocortical secretion in awake rats. Neuroendocrinology 59 , 97–109 (1994).
Buijs, R. M. et al. Anatomical and functional demonstration of a multisynaptic suprachiasmatic nucleus adrenal (cortex) pathway. Eur. J. Neurosci. 11 , 1535–1544 (1999).
Kiessling, S., Sollars, P. J. & Pickard, G. E. Light stimulates the mouse adrenal through a retinohypothalamic pathway independent of an effect on the clock in the suprachiasmatic nucleus. PLOS ONE 9 , e92959 (2014).
Husse, J., Leliavski, A., Tsang, A. H., Oster, H. & Eichele, G. The light-dark cycle controls peripheral rhythmicity in mice with a genetically ablated suprachiasmatic nucleus clock. FASEB J. 28 , 4950–4960 (2014).
Ishida, A. et al. Light activates the adrenal gland: timing of gene expression and glucocorticoid release. Cell Metab. 2 , 297–307 (2005).
Oster, H. et al. The circadian rhythm of glucocorticoids is regulated by a gating mechanism residing in the adrenal cortical clock. Cell Metab. 4 , 163–173 (2006).
Charmandari, E. et al. Peripheral CLOCK regulates target-tissue glucocorticoid receptor transcriptional activity in a circadian fashion in man. PLOS ONE 6 , e25612 (2011).
Bailey, S. L. & Heitkemper, M. M. Circadian rhythmicity of cortisol and body temperature: morningness-eveningness effects. Chronobiol. Int. 18 , 249–261 (2001).
Donner, N. C., Montoya, C. D., Lukkes, J. L. & Lowry, C. A. Chronic non-invasive corticosterone administration abolishes the diurnal pattern of tph2 expression. Psychoneuroendocrinology 37 , 645–661 (2012).
Lightman, S. L. The neuroendocrinology of stress: a never ending story. J. Neuroendocrinol. 20 , 880–884 (2008).
Nicolaides, N. C., Kyratzi, E., Lamprokostopoulou, A., Chrousos, G. P. & Charmandari, E. Stress, the stress system and the role of glucocorticoids. Neuroimmunomodulation 22 , 6–19 (2015).
Gibbison, B. et al. Dynamic pituitary-adrenal interactions in response to cardiac surgery. Crit. Care Med. 43 , 791–800 (2015).
Spiga, F. et al. Dynamic responses of the adrenal steroidogenic regulatory network. Proc. Natl Acad. Sci. USA 114 , E6466–E6474 (2017).
Ma, X. M., Levy, A. & Lightman, S. L. Emergence of an isolated arginine vasopressin (AVP) response to stress after repeated restraint: a study of both AVP and corticotropin-releasing hormone messenger ribonucleic acid (RNA) and heteronuclear RNA. Endocrinology 138 , 4351–4357 (1997).
Dallman, M. F. Stress update: adaptation of the hypothalamic-pituitary-adrenal axis to chronic stress. Trends. Endocrinol. Metab. 4 , 62–69 (1993).
Henley, D. E. et al. Hypothalamic-pituitary-adrenal axis activation in obstructive sleep apnea: the effect of continuous positive airway pressure therapy. J. Clin. Endocrinol. Metab. 94 , 4234–4242 (2009).
Boonen, E. et al. Reduced cortisol metabolism during critical illness. N. Engl. J. Med. 368 , 1477–1488 (2013).
Peeters, B. et al. Adrenocortical function during prolonged critical illness and beyond: a prospective observational study. Intensive Care Med. 44 , 1720–1729 (2018).
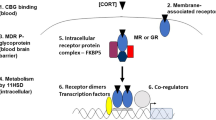
De Kloet, E. R., Vreugdenhil, E., Oitzl, M. S. & Joels, M. Brain corticosteroid receptor balance in health and disease. Endocr. Rev. 19 , 269–301 (1998).
Reul, J. M. & de Kloet, E. R. Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology 117 , 2505–2511 (1985).
Dallman, M. F. Fast glucocorticoid actions on brain: back to the future. Front. Neuroendocrinol. 26 , 103–108 (2005).
Russell, G. M. et al. Rapid glucocorticoid receptor-mediated inhibition of hypothalamic-pituitary-adrenal ultradian activity in healthy males. J. Neurosci. 30 , 6106–6115 (2010).
Lowenberg, M., Verhaar, A. P., van den Brink, G. R. & Hommes, D. W. Glucocorticoid signaling: a nongenomic mechanism for T cell immunosuppression. Trends Mol. Med. 13 , 158–163 (2007).
Orchinik, M., Murray, T. F., Franklin, P. H. & Moore, F. L. Guanyl nucleotides modulate binding to steroid receptors in neuronal membranes. Proc. Natl Acad. Sci. USA 89 , 3830–3834 (1992).
Orchinik, M., Murray, T. F. & Moore, F. L. A corticosteroid receptor in neuronal membranes. Science 252 , 1848–1851 (1991).
Joels, M., Pasricha, N. & Karst, H. The interplay between rapid and slow corticosteroid actions in brain. Eur. J. Pharmacol. 719 , 44–52 (2013).
Walker, J. J. et al. The origin of glucocorticoid hormone oscillations. PLOS Biol. 10 , e1001341 (2012).
Patel, P. D. et al. Glucocorticoid and mineralocorticoid receptor mRNA expression in squirrel monkey brain. J. Psychiatr. Res. 34 , 383–392 (2000).
Groeneweg, F. L., Karst, H., de Kloet, E. R. & Joels, M. Rapid non-genomic effects of corticosteroids and their role in the central stress response. J. Endocrinol. 209 , 153–167 (2011).
de Kloet, E. R., Fitzsimons, C. P., Datson, N. A., Meijer, O. C. & Vreugdenhil, E. Glucocorticoid signaling and stress-related limbic susceptibility pathway: about receptors, transcription machinery and microRNA. Brain Res. 1293 , 129–141 (2009).
Russell, G. M., Kalafatakis, K. & Lightman, S. L. The importance of biological oscillators for HPA activity and tissue glucocorticoid response: coordinating stress and neurobehavioural adaptation. J. Neuroendocrinol. 27 , 378–388 (2015).
Lewis, J. G. et al. Plasma variation of corticosteroid-binding globulin and sex hormone-binding globulin. Horm. Metab. Res. 38 , 241–245 (2006).
Lewis, J. G., Bagley, C. J., Elder, P. A., Bachmann, A. W. & Torpy, D. J. Plasma free cortisol fraction reflects levels of functioning corticosteroid-binding globulin. Clin. Chim. Acta 359 , 189–194 (2005).
Hammond, G. L., Smith, C. L. & Underhill, D. A. Molecular studies of corticosteroid binding globulin structure, biosynthesis and function. J. Steroid Biochem. Mol. Biol. 40 , 755–762 (1991).
Frairia, R. et al. Influence of naturally occurring and synthetic glucocorticoids on corticosteroid-binding globulin-steroid interaction in human peripheral plasma. Ann. NY Acad. Sci. 538 , 287–303 (1988).
Cameron, A. et al. Temperature-responsive release of cortisol from its binding globulin: a protein thermocouple. J. Clin. Endocrinol. Metab. 95 , 4689–4695 (2010).
Kyrou, I., Chrousos, G. P. & Tsigos, C. Stress, visceral obesity, and metabolic complications. Ann. NY Acad. Sci. 1083 , 77–110 (2006).
Chapman, K., Holmes, M. & Seckl, J. 11beta-hydroxysteroid dehydrogenases: intracellular gate-keepers of tissue glucocorticoid action. Physiol. Rev. 93 , 1139–1206 (2013).
Seckl, J. R. 11beta-hydroxysteroid dehydrogenases: changing glucocorticoid action. Curr. Opin. Pharmacol. 4 , 597–602 (2004).
Verma, M. et al. 11beta-hydroxysteroid dehydrogenase-1 deficiency alters brain energy metabolism in acute systemic inflammation. Brain Behav. Immun. 69 , 223–234 (2018).
Follenius, M., Simon, C., Brandenberger, G. & Lenzi, P. Ultradian plasma corticotropin and cortisol rhythms: time-series analyses. J. Endocrinol. Invest. 10 , 261–266 (1987).
Hartmann, A., Veldhuis, J. D., Deuschle, M., Standhardt, H. & Heuser, I. Twenty-four hour cortisol release profiles in patients with Alzheimer’s and Parkinson’s disease compared to normal controls: ultradian secretory pulsatility and diurnal variation. Neurobiol. Aging 18 , 285–289 (1997).
Rivest, R. W., Schulz, P., Lustenberger, S. & Sizonenko, P. C. Differences between circadian and ultradian organization of cortisol and melatonin rhythms during activity and rest. J. Clin. Endocrinol. Metab. 68 , 721–729 (1989).
Waite, E. J. et al. Ultradian corticosterone secretion is maintained in the absence of circadian cues. Eur. J. Neurosci. 36 , 3142–3150 (2012).
Ixart, G., Barbanel, G., Nouguier-Soule, J. & Assenmacher, I. A quantitative study of the pulsatile parameters of CRH-41 secretion in unanesthetized free-moving rats. Exp. Brain Res. 87 , 153–158 (1991).
Spiga, F. et al. ACTH-dependent ultradian rhythm of corticosterone secretion. Endocrinology 152 , 1448–1457 (2011).
Spiga, F., Liu, Y., Aguilera, G. & Lightman, S. L. Temporal effect of adrenocorticotrophic hormone on adrenal glucocorticoid steroidogenesis: involvement of the transducer of regulated cyclic AMP-response element-binding protein activity. J. Neuroendocrinol. 23 , 136–142 (2011).
Lim, C. & Allada, R. Emerging roles for post-transcriptional regulation in circadian clocks. Nat. Neurosci. 16 , 1544–1550 (2013).
Liston, C. et al. Circadian glucocorticoid oscillations promote learning-dependent synapse formation and maintenance. Nat. Neurosci. 16 , 698–705 (2013).
Lightman, S. L. & Conway-Campbell, B. L. The crucial role of pulsatile activity of the HPA axis for continuous dynamic equilibration. Nat. Rev. Neurosci. 11 , 710–718 (2010).
Stavreva, D. A. et al. Ultradian hormone stimulation induces glucocorticoid receptor-mediated pulses of gene transcription. Nat. Cell Biol. 11 , 1093–1102 (2009).
Conway-Campbell, B. L., Pooley, J. R., Hager, G. L. & Lightman, S. L. Molecular dynamics of ultradian glucocorticoid receptor action. Mol. Cell. Endocrinol. 348 , 383–393 (2012).
George, C. L., Lightman, S. L. & Biddie, S. C. Transcription factor interactions in genomic nuclear receptor function. Epigenomics 3 , 471–485 (2011).
So, A. Y., Chaivorapol, C., Bolton, E. C., Li, H. & Yamamoto, K. R. Determinants of cell- and gene-specific transcriptional regulation by the glucocorticoid receptor. PLOS Genet. 3 , e94 (2007).
Zalachoras, I., Houtman, R. & Meijer, O. C. Understanding stress-effects in the brain via transcriptional signal transduction pathways. Neuroscience 242 , 97–109 (2013).
Sarabdjitsingh, R. A. et al. Stress responsiveness varies over the ultradian glucocorticoid cycle in a brain-region-specific manner. Endocrinology 151 , 5369–5379 (2010).
Sarabdjitsingh, R. A. et al. Ultradian corticosterone pulses balance glutaminergic transmission and synaptic plasticity. Proc. Natl Acad. Sci. USA 111 , 14265–14270 (2014).
Kalafatakis, K. et al. Ultradian rhythmicity of plasma cortisol is necessary for normal emotional and cognitive responses in man. Proc. Natl Acad. Sci. USA 115 , E4091–E4100 (2018).
Gjerstad, J. K., Lightman, S. L. & Spiga, F. Role of glucocorticoid negative feedback in the regulation of HPA axis pulsatility. Stress 21 , 403–416 (2018).
Bornstein, S. R., Engeland, W. C., Ehrhart-Bornstein, M. & Herman, J. P. Dissociation of ACTH and glucocorticoids. Trends Endocrinol. Metab. 19 , 175–180 (2008).
Silverman, M. N., Miller, A. H., Biron, C. A. & Pearce, B. D. Characterization of an interleukin-6- and adrenocorticotropin-dependent, immune-to-adrenal pathway during viral infection. Endocrinology 145 , 3580–3589 (2004).
Franchimont, D. et al. Adrenal cortical activation in murine colitis. Gastroenterology 119 , 1560–1568 (2000).
Viblanc, V. A. et al. An integrative appraisal of the hormonal and metabolic changes induced by acute stress using king penguins as a model. Gen. Comp. Endocrinol. 269 , 1–10 (2018).
Cruz-Topete, D. & Cidlowski, J. A. One hormone, two actions: anti- and pro-inflammatory effects of glucocorticoids. Neuroimmunomodulation 22 , 20–32 (2015).
Biddie, S. C., Conway-Campbell, B. L. & Lightman, S. L. Dynamic regulation of glucocorticoid signalling in health and disease. Rheumatology 51 , 403–412 (2012).
Miller, G. E., Cohen, S. & Ritchey, A. K. Chronic psychological stress and the regulation of pro-inflammatory cytokines: a glucocorticoid-resistance model. Health Psychol. 21 , 531–541 (2002).
Oster, H. et al. The functional and clinical significance of the 24-hour rhythm of circulating glucocorticoids. Endocr. Rev. 38 , 3–45 (2017).
Keller, M. et al. A circadian clock in macrophages controls inflammatory immune responses. Proc. Natl Acad. Sci. USA 106 , 21407–21412 (2009).
Boivin, D. B. et al. Circadian clock genes oscillate in human peripheral blood mononuclear cells. Blood 102 , 4143–4145 (2003).
Koo, J. W., Russo, S. J., Ferguson, D., Nestler, E. J. & Duman, R. S. Nuclear factor-kappaB is a critical mediator of stress-impaired neurogenesis and depressive behavior. Proc. Natl Acad. Sci. USA 107 , 2669–2674 (2010).
Pace, T. W., Hu, F. & Miller, A. H. Cytokine-effects on glucocorticoid receptor function: relevance to glucocorticoid resistance and the pathophysiology and treatment of major depression. Brain Behav. Immun. 21 , 9–19 (2007).
Pace, T. W. et al. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. Am. J. Psychiatry 163 , 1630–1633 (2006).
Cohen, S. et al. Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk. Proc. Natl Acad. Sci. USA 109 , 5995–5999 (2012).
Spiegel, K., Leproult, R. & Van Cauter, E. Impact of sleep debt on metabolic and endocrine function. Lancet 354 , 1435–1439 (1999).
Hauner, H., Schmid, P. & Pfeiffer, E. F. Glucocorticoids and insulin promote the differentiation of human adipocyte precursor cells into fat cells. J. Clin. Endocrinol. Metab. 64 , 832–835 (1987).
Dallman, M. F. et al. Glucocorticoids, chronic stress, and obesity. Prog. Brain Res. 153 , 75–105 (2006).
Tsigos, C. et al. Dose-dependent effects of recombinant human interleukin-6 on glucose regulation. J. Clin. Endocrinol. Metab. 82 , 4167–4170 (1997).
McEwen, B. S. Sleep deprivation as a neurobiologic and physiologic stressor: allostasis and allostatic load. Metabolism 55 , S20–S23 (2006).
Zhu, B., Shi, C., Park, C. G., Zhao, X. & Reutrakul, S. Effects of sleep restriction on metabolism-related parameters in healthy adults: a comprehensive review and meta-analysis of randomized controlled trials. Sleep Med. Rev. 45 , 18–30 (2019).
Gavrila, A. et al. Diurnal and ultradian dynamics of serum adiponectin in healthy men: comparison with leptin, circulating soluble leptin receptor, and cortisol patterns. J. Clin. Endocrinol. Metab. 88 , 2838–2843 (2003).
Knutson, K. L. & Van Cauter, E. Associations between sleep loss and increased risk of obesity and diabetes. Ann. NY Acad. Sci. 1129 , 287–304 (2008).
Adam, T. C. & Epel, E. S. Stress, eating and the reward system. Physiol. Behav. 91 , 449–458 (2007).
Young, E. A., Carlson, N. E. & Brown, M. B. Twenty-four-hour ACTH and cortisol pulsatility in depressed women. Neuropsychopharmacology 25 , 267–276 (2001).
Heuser, I., Yassouridis, A. & Holsboer, F. The combined dexamethasone/CRH test: a refined laboratory test for psychiatric disorders. J. Psychiatr. Res. 28 , 341–356 (1994).
Ising, M. et al. The combined dexamethasone/CRH test as a potential surrogate marker in depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 29 , 1085–1093 (2005).
Krishnan, V. & Nestler, E. J. The molecular neurobiology of depression. Nature 455 , 894–902 (2008).
Miller, A. H. Depression and immunity: a role for T cells? Brain Behav. Immun. 24 , 1–8 (2010).
Koo, J. W. & Duman, R. S. IL-1beta is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc. Natl Acad. Sci. USA 105 , 751–756 (2008).
Horowitz, M. A., Zunszain, P. A., Anacker, C., Musaelyan, K. & Pariante, C. M. Glucocorticoids and inflammation: a double-headed sword in depression? How do neuroendocrine and inflammatory pathways interact during stress to contribute to the pathogenesis of depression? Mod. Trends Pharmacopsychiatry 28 , 127–143 (2013).
Munhoz, C. D. et al. Chronic unpredictable stress exacerbates lipopolysaccharide-induced activation of nuclear factor-kappaB in the frontal cortex and hippocampus via glucocorticoid secretion. J. Neurosci. 26 , 3813–3820 (2006).
Pariante, C. M. Glucocorticoid receptor function in vitro in patients with major depression. Stress 7 , 209–219 (2004).
Kenis, G. & Maes, M. Effects of antidepressants on the production of cytokines. Int. J. Neuropsychopharmacol. 5 , 401–412 (2002).
Bjornsdottir, S. et al. Drug prescription patterns in patients with Addison’s disease: a Swedish population-based cohort study. J. Clin. Endocrinol. Metab. 98 , 2009–2018 (2013).
Dunlop, D. Eighty-six cases of Addison’s disease. BMJ 2 , 887–891 (1963).
Giordano, R. et al. Metabolic and cardiovascular profile in patients with Addison’s disease under conventional glucocorticoid replacement. J. Endocrinol. Invest. 32 , 917–923 (2009).
Johannsson, G. et al. Adrenal insufficiency: review of clinical outcomes with current glucocorticoid replacement therapy. Clin. Endocrinol. 82 , 2–11 (2015).
Lovas, K., Loge, J. H. & Husebye, E. S. Subjective health status in Norwegian patients with Addison’s disease. Clin. Endocrinol. 56 , 581–588 (2002).
Feek, C. M. et al. Patterns of plasma cortisol and ACTH concentrations in patients with Addison’s disease treated with conventional corticosteroid replacement. Clin. Endocrinol. 14 , 451–458 (1981).
Isidori, A. M. et al. Effect of once-daily, modified-release hydrocortisone versus standard glucocorticoid therapy on metabolism and innate immunity in patients with adrenal insufficiency (DREAM): a single-blind, randomised controlled trial. Lancet Diabetes Endocrinol. 6 , 173–185 (2018).
Bancos, I. et al. Primary adrenal insufficiency is associated with impaired natural killer cell function: a potential link to increased mortality. Eur. J. Endocrinol. 176 , 471–480 (2017).
Bjanesoy, T. E. et al. Altered DNA methylation profile in Norwegian patients with autoimmune Addison’s disease. Mol. Immunol. 59 , 208–216 (2014).
Langenheim, J., Ventz, M., Hinz, A. & Quinkler, M. Modified-release prednisone decreases complaints and fatigue compared to standard prednisolone in patients with adrenal insufficiency. Horm. Metab. Res. 45 , 96–101 (2013).
Mallappa, A. et al. A phase 2 study of Chronocort, a modified-release formulation of hydrocortisone, in the treatment of adults with classic congenital adrenal hyperplasia. J. Clin. Endocrinol. Metab. 100 , 1137–1145 (2015).
Lovas, K. & Husebye, E. S. Continuous subcutaneous hydrocortisone infusion in Addison’s disease. Eur. J. Endocrinol. 157 , 109–112 (2007).
Venneri, M. A. et al. Circadian rhythm of glucocorticoid administration entrains clock genes in immune cells: a DREAM trial ancillary study. J. Clin. Endocrinol. Metab. 103 , 2998–3009 (2018).
Oksnes, M. et al. Continuous subcutaneous hydrocortisone infusion versus oral hydrocortisone replacement for treatment of Addison’s disease: a randomized clinical trial. J. Clin. Endocrinol. Metab. 99 , 1665–1674 (2014).
Riedel, M., Wiese, A., Schurmeyer, T. H. & Brabant, G. Quality of life in patients with Addison’s disease: effects of different cortisol replacement modes. Exp. Clin. Endocrinol. 101 , 106–111 (1993).
van Staa, T. P. et al. Use of oral corticosteroids in the United Kingdom. QJM 93 , 105–111 (2000).
Overman, R. A., Yeh, J. Y. & Deal, C. L. Prevalence of oral glucocorticoid usage in the United States: a general population perspective. Arthritis Care Res. 65 , 294–298 (2013).
Curtis, J. R. et al. Population-based assessment of adverse events associated with long-term glucocorticoid use. Arthritis Rheum. 55 , 420–426 (2006).
McDonough, A. K., Curtis, J. R. & Saag, K. G. The epidemiology of glucocorticoid-associated adverse events. Curr. Opin. Rheumatol. 20 , 131–137 (2008).
Leung, D. Y. & Bloom, J. W. Update on glucocorticoid action and resistance. J. Allergy Clin. Immunol. 111 , 3–22 (2003).
Download references
Author information
Authors and affiliations.
Translational Health Sciences, Dorothy Hodgkin Building, Bristol Medical School, University of Bristol, Bristol, UK
Georgina Russell & Stafford Lightman
You can also search for this author in PubMed Google Scholar
Contributions
The authors contributed equally to all aspects of the article.
Corresponding authors
Correspondence to Georgina Russell or Stafford Lightman .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Cues that entrain or synchronize the body’s 24-h cycle
Biological rhythms that occur with a frequency of <24 h.
A biochemical oscillator with phases synchronized with solar time.
Neural pathways involving at least one relay.
The microcirculation that allows transport of hypothalamic hormones to the pituitary gland.
The threshold power of (solar) electromagnetic radiation needed to exert an effect.
Repetitive body movements that serve no biological function.
Behaviours engaged for a specific functional purpose.
Any biological process that displays an oscillation of approximately 24 h.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Russell, G., Lightman, S. The human stress response. Nat Rev Endocrinol 15 , 525–534 (2019). https://doi.org/10.1038/s41574-019-0228-0
Download citation
Accepted : 05 June 2019
Published : 27 June 2019
Issue Date : September 2019
DOI : https://doi.org/10.1038/s41574-019-0228-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Evaluation of the benefits of neutral bicarbonate ionized water baths in an open-label, randomized, crossover trial.
- Ryoko Ushikoshi-Nakayama
- Tomoe Yamazaki
- Ichiro Saito
Scientific Reports (2024)
Sex-specific associations of serum cortisol with brain biomarkers of Alzheimer’s risk
- Lisa Mosconi
- Schantel Williams
- Jonathan P. Dyke
Evaluation of indicators of acute emotional states in dogs
- Hannah E. Flint
- Jennifer E. Weller
- Tammie King
Early Childhood Education Teacher Workforce: Stress in Relation to Identity and Choices
- Cynthia A. Wiltshire
Early Childhood Education Journal (2024)
Association between maltreatment, hair cortisol concentration, positive parent–child interaction, and psychosocial outcomes in Chinese preschool children
- Wenjie Shan
- Yunting Zhang
European Child & Adolescent Psychiatry (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Stress Management
Affiliations.
- 1 University of Louisville
- 2 Univ of Louisville School of Medicine
- PMID: 30020672
- Bookshelf ID: NBK513300
Effective techniques for stress management are varied. They typically include behaviors that improve physical health, such as nutrition and exercise, but may also incorporate strategies that improve cognitive and emotional functioning. The stress-reduction approach based on mindfulness practices has recently enjoyed an explosion of interest from a variety of healthcare and epidemiological researchers. The concept of mindfulness, which originates from practices of Buddhism, is defined as a focused awareness of one’s experience, and purposeful and nonjudgmental focus on the present moment. Structured interventions, such as the Mindfulness-Based Stress Reduction (MBSR) program, provide participants with the opportunity to learn breathing meditation, body scanning techniques, and gentle, yoga-inspired physical exercises. With practice, individuals learn to process emotions, thoughts, and sensations as they arise. Individuals learn to modify their reflexive conditioning from automatically reacting or worrying about the future to a more adaptive, measured response with greater awareness of the present moment. The literature is replete with evidence suggesting that, with practice, individuals can become more mindful, increasing their capacity to fully process emotions, thoughts, and sensations as they arise. MBSR interventions have been adapted to a wide variety of individuals, from those suffering from chronic or debilitating health conditions to healthy undergraduate or medical students. Randomized controlled trials of MBSR interventions have demonstrated improvements to psychological and physiological processes with relevance to health outcomes and improved stress management.
Some individuals have a greater innate, or trait, capacity for mindfulness. These individuals, who have not participated in mindfulness-training interventions, tend to experience better physical health, report fewer physiological symptoms such as pain, and utilize fewer healthcare resources. Trait mindfulness has been associated with lower ratings of anxiety and depression in a variety of medical and non-medical populations. Trait mindfulness may emerge from a genetic predisposition. A recent epidemiological study of adolescent twins revealed that trait mindfulness was 32% heritable. The same study also revealed that 66% of the variance in trait mindfulness was due to environmental factors, suggesting that is also a skill that can be learned. In fact, an MBSR study in university undergraduates revealed that, while increases in mindfulness and psychological outcomes can be observed in participants as a whole, effects may be more pronounced among individuals higher in trait mindfulness at study entry. These data substantiate the utility of mindfulness training, even for high-trait individuals.
Copyright © 2024, StatPearls Publishing LLC.
- Introduction
- Issues of Concern
- Clinical Significance
- Review Questions
Publication types
- Study Guide
- Research Article
- Open access
- Published: 06 April 2021
Health anxiety, perceived stress, and coping styles in the shadow of the COVID-19
- Szabolcs Garbóczy 1 , 2 ,
- Anita Szemán-Nagy 3 ,
- Mohamed S. Ahmad 4 ,
- Szilvia Harsányi 1 ,
- Dorottya Ocsenás 5 , 6 ,
- Viktor Rekenyi 4 ,
- Ala’a B. Al-Tammemi 1 , 7 &
- László Róbert Kolozsvári ORCID: orcid.org/0000-0001-9426-0898 1 , 7
BMC Psychology volume 9 , Article number: 53 ( 2021 ) Cite this article
15k Accesses
41 Citations
Metrics details
In the case of people who carry an increased number of anxiety traits and maladaptive coping strategies, psychosocial stressors may further increase the level of perceived stress they experience. In our research study, we aimed to examine the levels of perceived stress and health anxiety as well as coping styles among university students amid the COVID-19 pandemic.
A cross-sectional study was conducted using an online-based survey at the University of Debrecen during the official lockdown in Hungary when dormitories were closed, and teaching was conducted remotely. Our questionnaire solicited data using three assessment tools, namely, the Perceived Stress Scale (PSS), the Ways of Coping Questionnaire (WCQ), and the Short Health Anxiety Inventory (SHAI).
A total of 1320 students have participated in our study and 31 non-eligible responses were excluded. Among the remaining 1289 participants, 948 (73.5%) and 341 (26.5%) were Hungarian and international students, respectively. Female students predominated the overall sample with 920 participants (71.4%). In general, there was a statistically significant positive relationship between perceived stress and health anxiety. Health anxiety and perceived stress levels were significantly higher among international students compared to domestic ones. Regarding coping, wishful thinking was associated with higher levels of stress and anxiety among international students, while being a goal-oriented person acted the opposite way. Among the domestic students, cognitive restructuring as a coping strategy was associated with lower levels of stress and anxiety. Concerning health anxiety, female students (domestic and international) had significantly higher levels of health anxiety compared to males. Moreover, female students had significantly higher levels of perceived stress compared to males in the international group, however, there was no significant difference in perceived stress between males and females in the domestic group.
The elevated perceived stress levels during major life events can be further deepened by disengagement from home (being away/abroad from country or family) and by using inadequate coping strategies. By following and adhering to the international recommendations, adopting proper coping methods, and equipping oneself with the required coping and stress management skills, the associated high levels of perceived stress and anxiety could be mitigated.
Peer Review reports
Introduction
On March 4, 2020, the first cases of coronavirus disease were declared in Hungary. One week later, the World Health Organization (WHO) declared COVID-19 as a global pandemic [ 1 ]. The Hungarian government ordered a ban on outdoor public events with more than 500 people and indoor events with more than 100 participants to reduce contact between people [ 2 ]. On March 27, the government imposed a nationwide lockdown for two weeks effective from March 28, to mitigate the spread of the pandemic. Except for food stores, drug stores, pharmacies, and petrol stations, all other shops and educational institutions remained closed. On April 16, a week-long extension was further announced [ 3 ].
The COVID-19 pandemic with its high morbidity and mortality has already afflicted the psychological and physical wellbeing of humans worldwide [ 4 , 5 , 6 , 7 , 8 , 9 ]. During major life events, people may have to deal with more stress. Stress can negatively affect the population’s well-being or function when they construe the situation as stressful and they cannot handle the environmental stimuli [ 10 ]. Various inter-related and inter-linked concepts are present in such situations including stress, anxiety, and coping. According to the literature, perceived stress can lead to higher levels of anxiety and lower levels of health-related quality of life [ 11 ]. Another study found significant and consistent associations between coping strategies and the dimensions of health anxiety [ 12 ].
Health anxiety is one of the most common types of anxiety and it describes how people think and behave toward their health and how they perceive any health-related concerns or threats. Health anxiety is increasingly conceptualized as existing on a spectrum [ 13 , 14 ], and as an adaptive signal that helps to develop survival-oriented behaviors. It also occurs in almost everyone’s life to a certain degree and can be rather deleterious when it is excessive [ 13 , 14 ]. Illness anxiety or hypochondriasis is on the high end of the spectrum and it affects someone’s life when it interferes with daily life by making people misinterpret the somatic sensations, leading them to think that they have an underlying condition [ 14 ].
According to the American Psychiatric Association—Diagnostic and Statistical Manual of Mental Disorders (fifth edition), Illness anxiety disorder is described as a preoccupation with acquiring or having a serious illness, and it reflects the high spectrum of health anxiety [ 15 ]. Somatic symptoms are not present or if they are, then only mild in intensity. The preoccupation is disproportionate or excessive if there is a high risk of developing a medical condition (e.g., family history) or the patient has another medical condition. Excessive health-related behaviors can be observed (e.g., checking body for signs of illness) and individuals can show maladaptive avoidance as well by avoiding hospitals and doctor appointments [ 15 ].
Health anxiety is indeed an important topic as both its increase and decrease can progress to problems [ 14 ]. Looking at health anxiety as a wide spectrum, it can be high or low [ 16 ]. While people with a higher degree of worry and checking behaviors may cause some burden on healthcare facilities by visiting them too many times (e.g., frequent unnecessary visits), other individuals may not seek medical help at healthcare units to avoid catching up infections for instance. A lower degree of health anxiety can lead to low compliance with imposed regulations made to control a pandemic [ 17 ].
The COVID-19 pandemic as a major event in almost everyone’s life has posed a great impact on the population’s perceived stress level. Several studies about the relation between coping and response to epidemics in recent and previous outbreaks found higher perceived stress levels among people [ 18 , 19 , 20 , 21 ]. Being a woman, low income, and living with other people all were associated with higher stress levels [ 18 ]. Protective factors like being emotionally more stable, having self-control, adaptive coping strategies, and internal locus of control were also addressed [ 19 , 20 ]. The findings indicated that the COVID-19 crisis is perceived as a stressful event. The perceived stress was higher amongst people than it was in situations with no emergency. Nervousness, stress, and loss of control of one’s life are the factors that are most connected to perceived stress levels which leads to the suggestion that unpredictability and uncontrollability take an important part in perceived stress during a crisis [ 19 , 20 ].
Moreover, certain coping styles (e.g., having a positive attitude) were associated with less psychological distress experiences but avoidance strategies were more likely to cause higher levels of stress [ 21 ]. According to Lazarus (1999), individuals differ in their perception of stress if the stress response is viewed as the interaction between the environment and humans [ 22 ]. An Individual can experience two kinds of evaluation processes, one to appraise the external stressors and personal stake, and the other one to appraise personal resources that can be used to cope with stressors [ 22 , 23 ]. If there is an imbalance between these two evaluation processes, then stress occurs, because the personal resources are not enough to cope with the stressor’s demands [ 23 ].
During stressful life events, it is important to pay attention to the increasing levels of health anxiety and to the kind of coping mechanisms that are potential factors to mitigate the effects of high anxiety. The transactional model of stress by Lazarus and Folkman (1987) provides an insight into these kinds of factors [ 24 ]. Lazarus and Folkman theorized two types of coping responses: emotion-focused coping, and problem-focused coping. Emotion-focused coping strategies (e.g., distancing, acceptance of responsibility, positive reappraisal) might be used when the source of stress is not embedded in the person’s control and these strategies aim to manage the individual’s emotional response to a threat. Also, emotion-focused coping strategies are directed at managing emotional distress [ 24 ]. On the other hand, problem-focused coping strategies (e.g., confrontive coping, seeking social support, planful problem-solving) help an individual to be able to endure and/or minimize the threat, targeting the causes of stress in practical ways [ 24 ]. It was also addressed that emotion-focused coping mechanisms were used more in situations appraised as requiring acceptance, whereas problem-focused forms of coping were used more in encounters assessed as changeable [ 24 ].
A recent study in Hunan province in China found that the most effective factor in coping with stress among medical staff was the knowledge of their family’s well-being [ 25 ]. Although there have been several studies about the mental health of hospital workers during the COVID-19 pandemic or other epidemics (e.g., SARS, MERS) [ 26 , 27 , 28 , 29 ], only a few studies from recent literature assessed the general population’s coping strategies. According to Gerhold (2020) [ 30 ], older people perceived a lower risk of COVID-19 than younger people. Also, women have expressed more worries about the disease than men did. Coping strategies were highly problem-focused and most of the participants reported that they listen to professionals’ advice and tried to remain calm [ 30 ]. In the same study, most responders perceived the COVID-19 pandemic as a global catastrophe that will severely affect a lot of people. On the other hand, they perceived the pandemic as a controllable risk that can be reduced. Dealing with macrosocial stressors takes faith in politics and in those people, who work with COVID-19 on the frontline.
Mental disorders are found prevalent among college students and their onset occurs mostly before entry to college [ 31 ]. The diagnosis and timely interventions at an early stage of illness are essential to improve psychosocial functioning and treatment outcomes [ 31 ]. According to research that was conducted at the University of Debrecen in Hungary a few years ago, the students were found to have high levels of stress and the rate of the participants with impacted mental health was alarming [ 32 ]. With an unprecedented stressful event like the COVID-19 crisis, changes to the mental health status of people, including students, are expected.
Aims of the study
In our present study, we aimed at assessing the levels of health anxiety, perceived stress, and coping styles among university students amidst the COVID-19 lockdown in Hungary, using three validated assessment tools for each domain.
Methods and materials
Study design and setting.
This study utilized a cross-sectional design, using online self-administered questionnaires that were created and designed in Google Forms® (A web-based survey tool). Data collection was carried out in the period April 30, 2020, and May 15, 2020, which represents one of the most stressful periods during the early stage of the COVID-19 pandemic in Hungary when the official curfew/lockdown was declared along with the closure of dormitories and shifting to online remote teaching. The first cases of COVID-19 were declared in Hungary on March 4, 2020. On April 30, 2020, there were 2775 confirmed cases, 312 deaths, and 581 recoveries. As of May 15, 2020, the number of confirmed cases, deaths, and recovered persons was 3417, 442, and 1287, respectively.
Our study was conducted at the University of Debrecen, which is one of the largest higher education institutions in Hungary. The University is located in the city of Debrecen, the second-largest city in Hungary. Debrecen city is considered the educational and cultural hub of Eastern Hungary. As of October 2019, around 28,593 students were enrolled in various study programs at the University of Debrecen, of whom, 6,297 were international students [ 33 ]. The university offers various degree courses in Hungarian and English languages.
Study participants and sampling
The target population of our study was students at the University of Debrecen. Students were approached through social media platforms (e.g., Facebook®) and the official student administration system at the University of Debrecen (Neptun). The invitation link to our survey was sent to students on the web-based platforms described earlier. By using the Neptun system, we theoretically assumed that our survey questionnaire has reached all students at the University. The students who were interested and willing to participate in the study could fill out our questionnaire anonymously during the determined study period; thus, employing a convenience sampling approach. All students at the University of Debrecen whose age was 18 years or older and who were in Hungary during the outbreak had the eligibility to participate in our study whether undergraduates or postgraduates.
Study instruments
In our present study, the survey has solicited information about the sociodemographic profile of participants including age (in years), gender (female vs male), study program (health-related vs non-health related), and whether the student stayed in Hungary or traveled abroad during the period of conducting our survey in the outbreak. Our survey has also adopted three international scales to collect data about health anxiety, coping styles, and perceived stress during the pandemic crisis. As the language of instruction for international students at the University of Debrecen is English, and English fluency is one of the criteria for international students’ admission at the University of Debrecen, the international students were asked to fill out the English version of the survey and the scales. On the other hand, the Hungarian students were asked to fill out the Hungarian version of the survey and the validated Hungarian scales. Also, we provided contact information for psychological support when needed. Students who felt that they needed some help and psychological counseling could use the contact information of our peer supporters. Four International students have used this opportunity and were referred to a higher level of care. The original scales and their validated Hungarian versions are described in the following sections.
Perceived Stress Scale (PSS)
The Perceived Stress Scale (PSS) measures the level of stress in the general population who have at least completed a junior high school [ 34 ]. In the PSS, the respondents had to report how often certain things occurred like nervousness; loss of control; feeling of upset; piling up difficulties that cannot be handled; or on the contrary how often the students felt they were able to handle situations; and were on top of things. For the International students, we used the 10-item PSS (English version). The statements’ responses were scored on a 5-point Likert scale (from 0 = never to 4 = very often) as per the scale’s guide. Also, in the 10-item PSS, four positive items were reversely scored (e.g. felt confident about someone’s ability to handle personal problems) [ 34 ]. The PSS has satisfactory psychometric properties with a Cronbach’s alpha of 0.78, and this English version was used for international students in our study.
For the Hungarian students, we used the Hungarian version of the PSS, which has 14 statements that cover the same aspects of stress described earlier. In this version of the PSS, the responses were evaluated on a 5-point Likert scale (0–4) to mark how typical a particular behavior was for a respondent in the last month [ 35 ]. The Hungarian version of the PSS was psychometrically validated in 2006. In the validation study, the Hungarian 14-item PSS has shown satisfactory internal consistency with a Cronbach’s alpha of 0.88 [ 35 ].
Ways of Coping Questionnaire (WCQ)
The second scale we used was the 26-Item Ways of Coping Questionnaire (WCQ) which was developed by Sørlie and Sexton [ 36 ]. For the international students, we used the validated English version of the 26-Item WCQ that distinguished five different factors, including Wishful thinking (hoped for a miracle, day-dreamed for a better time), Goal-oriented (tried to analyze the problem, concentrated on what to do), Seeking support (talked to someone, got professional help), Thinking it over (drew on past experiences, realized other solutions), and Avoidance (refused to think about it, minimized seriousness of it). The WCQ examined how often the respondents used certain coping mechanisms, eg: hoped for a miracle, fantasized, prepared for the worst, analyzed the problem, talked to someone, or on the opposite did not talk to anyone, drew conclusions from past things, came up with several solutions for a problem or contained their feelings. As per the 26-item WCQ, responses were scored on a 4-point Likert scale (from 0 = “does not apply and/or not used” to 3 = “used a great deal”). This scale has satisfactory psychometric properties with Cronbach's alpha for the factors ranged from 0.74 to 0.81[ 36 ].
For the Hungarian students, we used the Hungarian 16-Item WCQ, which was validated in 2008 [ 37 ]. In the Hungarian WCQ, four dimensions were identified, which were cognitive restructuring/adaptation (every cloud has a silver lining), Stress reduction (by eating; drinking; smoking), Problem analysis (I tried to analyze the problem), and Helplessness/Passive coping (I prayed; used drugs) [ 37 ]. The Cronbach’s alpha values for the Hungarian WCQ’s dimensions were in the range of 0.30–0.74 [ 37 ].
Short Health Anxiety Inventory (SHAI)
The third scale adopted was the 18-Items Short Health Anxiety Inventory (SHAI). Overall, the SHAI has two subscales. The first subscale comprised of 14 items that examined to what degree the respondents were worried about their health in the past six months; how often they noticed physical pain/ache or sensations; how worried they were about a serious illness; how much they felt at risk for a serious illness; how much attention was drawn to bodily sensations; what their environment said, how much they deal with their health. The second subscale of SHAI comprised of 4 items (negative consequences if the illness occurs) that enquired how the respondents would feel if they were diagnosed with a serious illness, whether they would be able to enjoy things; would they trust modern medicine to heal them; how many aspects of their life it would affect; how much they could preserve their dignity despite the illness [ 38 ]. One of four possible statements (scored from 0 to 3) must be chosen. Alberts et al. (2013) [ 39 ] found the mean SHAI value to be 12.41 (± 6.81) in a non-clinical sample. The original 18-item SHAI has Cronbach’s alpha values in the range of 0.74–0.96 [ 39 ]. For the Hungarian students, the Hungarian version of the SHAI was used. The Hungarian version of SHAI was validated in 2011 [ 40 ]. The scoring differs from the English version in that the four statements were scored from 1 to 4, but the statements themselves were the same. In the Hungarian validation study, it was found that the SHAI mean score in a non-clinical sample (university students) was 33.02 points (± 6.28) and the Cronbach's alpha of the test was 0.83 [ 40 ].
Data analyses
Data were extracted from Google Forms® as an Excel sheet for quality check and coding then we used SPSS® (v.25) and RStudio statistical software packages to analyze the data. Descriptive and summary statistics were presented as appropriate. To assess the difference between groups/categories of anxiety, stress, and coping styles, we used the non-parametric Kruskal–Wallis test, since the variables did not have a normal distribution and for post hoc tests, we used the Mann–Whitney test. Also, we used Spearman’s rank correlation to assess the relationship between health anxiety and perceived stress within the international group and the Hungarian group. Comparison between international and domestic groups and different genders in terms of health anxiety and perceived stress levels were also conducted using the Mann–Whitney test. Binary logistic regression analysis was also employed to examine the associations between different coping styles/ strategies (treated as independent variables) and both, health anxiety level and perceived stress level (treated as outcome variables) using median splits. A p-value less than 5% was implemented for statistical significance.
Ethical considerations
Ethical permission was obtained from the Hungarian Ethical Review Committee for Research in Psychology (Reference number: 2020-45). All methods were carried out following the institutional guidelines and conforming to the ethical standards of the declaration of Helsinki. All participants were informed about the study and written informed consent was obtained before completing the survey. There were no rewards/incentives for completing the survey.
Sociodemographic characteristics of respondents
A total of 1320 students have responded to our survey. Six responses were eliminated due to incompleteness and an additional 25 responses were also excluded as the students filled out the survey from abroad (International students who were outside Hungary during the period of conducting our study). After exclusion of the described non-eligible responses (a total of 31 responses), the remaining 1289 valid responses were included in our analysis. Out of 1289 participants (100%), 73.5% were Hungarian students and around 26.5% were international students. Overall, female students have predominated the sample (n = 920, 71.4%). The median age (Interquartile range) among Hungarian students was 22 years (5) and for the international students was 22 years (4). Out of the total sample, most of the Hungarian students were enrolled in non-health-related programs (n = 690, 53.5%), while most of the international students were enrolled in health-related programs (n = 213, 16.5%). Table 1 demonstrates the sociodemographic profile of participants (Hungarian vs International).
Perceived stress, anxiety, and coping styles
For greater clarity of statistical analysis and interpretation, we created preferences regarding coping mechanisms. That is, we made the categories based on which coping factor (in the international sample) or dimension (in the Hungarian sample) the given person reached the highest scores, so it can be said that it is the person's preferred coping strategy. The four coping strategies among international students were goal-oriented, thinking it over, wishful thinking, and avoidance, while among the Hungarian students were cognitive restructuring, problem analysis, stress reduction, and passive coping.
The 26-item WCQ [ 31 ] contains a seeking support subscale which is missing from the Hungarian 16-item WCQ [ 32 ]; therefore, the seeking support subscale was excluded from our analysis. Moreover, because the PSS contained a different number of items in English and Hungarian versions (10 items vs 14 items), we looked at the average score of the answers so that we could compare international and domestic students.
In the evaluation of SHAI, the scoring of the two questionnaires are different. For the sake of comparability between the two samples, the international points were corrected to the Hungarian, adding plus one to the value of each answer. This may be the reason why we obtained higher results compared to international standards.
Among the international students, the mean score (± standard deviation) of perceived stress among male students was 2.11(± 0.86) compared to female students 2.51 (± 0.78), while the mean score (± standard deviation) of health anxiety was 34.12 (± 7.88) and 36.31 (± 7.75) among males and females, respectively. Table 2 shows more details regarding the perceived stress scores and health anxiety scores stratified by coping strategies among international students.
In the Hungarian sample, the mean score (± standard deviation) of perceived stress among male students was 2.06 (± 0.84) compared to female students 2.18 (± 0.83), while the mean score (± standard deviation) of health anxiety was 33.40 (± 7.63) and 35.05 (± 7.39) among males and females, respectively. Table 3 shows more details regarding the perceived stress scores and health anxiety scores stratified by coping strategies among Hungarian students.
Concerning coping styles among international students, the statements with the highest-ranked responses were “wished the situation would go away or somehow be finished” and “Had fantasies or wishes about how things might turn out” and both fall into the wishful thinking coping. Among the Hungarian students, the statements with the highest-ranked responses were “I tried to analyze the problem to understand better” (falls into problem analysis coping) and “I thought every cloud has a silver lining, I tried to perceive things cheerfully” (falls into cognitive restructuring coping).
On the other hand, the statements with the least-ranked responses among the international students belonged to the Avoidance coping. Among the Hungarians, it was Passive coping “I tried to take sedatives or medications” and Stress reduction “I staked everything upon a single cast, I started to do something risky” to have the lowest-ranked responses. Table 4 shows a comparison of different coping strategies among international and Hungarian students.
To test the difference between coping strategies, we used the non-parametric Kruskal–Wallis test, since the variables did not have a normal distribution. For post hoc tests, we used Mann–Whitney tests with lowered significance levels ( p = 0.0083). Among Hungarian students, there were significant differences between the groups in stress ( χ 2 (3) = 212.01; p < 0.001) and health anxiety ( χ 2 (3) = 80.32; p < 0.001). In the post hoc tests, there were significant differences everywhere ( p < 0.001) except between stress reduction and passive coping ( p = 0.089) and between problem analysis and passive coping ( p = 0.034). Considering the health anxiety, the results were very similar. There were significant differences between all groups ( p < 0.001), except between stress reduction and passive coping ( p = 0.347) and between problem analysis and passive coping ( p = 0.205). See Figs. 1 and 2 for the Hungarian students.
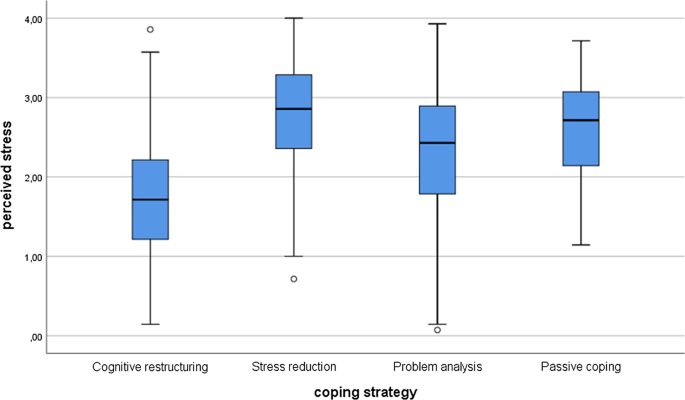
Perceived stress differences between coping strategies among the Hungarian students
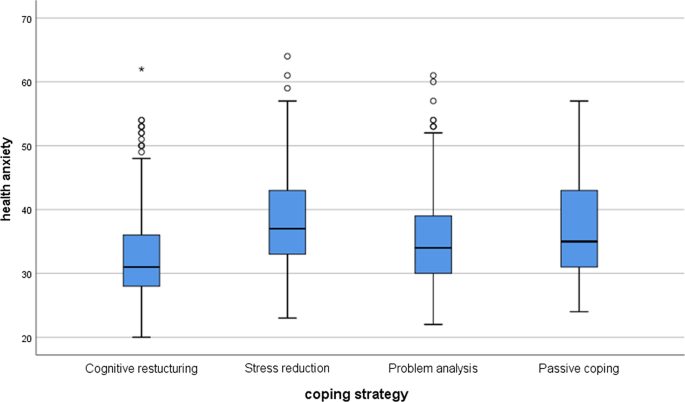
Health anxiety differences between coping strategies among the Hungarian students
Among the international students, the results were similar. According to the Kruskal–Wallis test, there were significant differences in stress ( χ 2 (3) = 73.26; p < 0.001) and health anxiety ( χ 2 (3) = 42.60; p < 0.001) between various coping strategies. The post hoc tests showed that there were differences between the perceived stress level and coping strategies everywhere ( p < 0.005) except and between avoidance and thinking it over ( p = 0.640). Concerning health anxiety, there were significant differences between wishful thinking and goal-oriented ( p < 0.001), between wishful thinking and avoidance ( p = 0.001), and between goal-oriented and avoidance ( p = 0.285). There were no significant differences between wishful thinking and thinking it over ( p = 0.069), between goal-oriented and thinking it over ( p = 0.069), and between avoidance and thinking it over ( p = 0.131). See Figs. 3 and 4 .
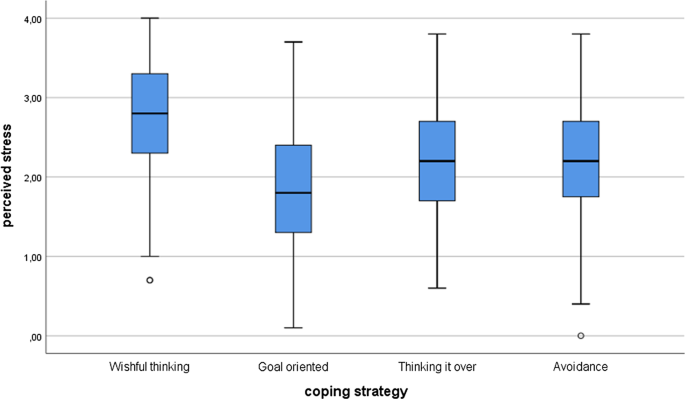
Perceived stress differences between coping strategies among the international students
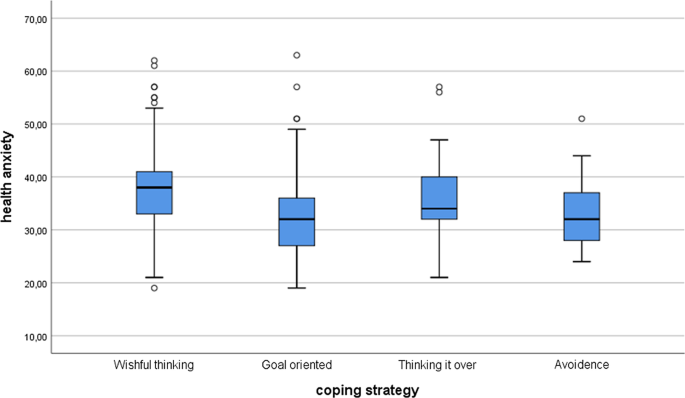
Health anxiety differences between coping strategies among the international students
The relationship between coping strategies with health anxiety and perceived stress levels among the international students
We applied logistic regression analyses for the variables to see which of the coping strategies has a significant effect on SHAI and PSS results. In the first model (model a), with the health anxiety as an outcome dummy variable (with median split; median: 35), only two coping strategies had a statistically significant relationship with health anxiety level, including wishful thinking (as a risk factor) and goal-oriented (as a protective factor).
In the second model (model b), with the perceived stress as an outcome dummy variable (with median split; median: 2.40), three coping strategies were found to have a statistically significant association with the level of perceived stress, including wishful thinking (as a risk factor), while goal-oriented and thinking it over as protective factors. See Table 5 .
The relationship between coping strategies with health anxiety and perceived stress levels among domestic students
By employing logistic regression analysis, with the health anxiety as an outcome dummy variable (with median split; median: 33.5) (model a), three coping strategies had a statistically significant relationship with health anxiety level among domestic students, including stress reduction and problem analysis (as risk factors), while cognitive restructuring (as a protective factor).
Similarly, with the perceived stress as an outcome dummy variable (with median split; median: 2.1429) (model b), three coping strategies had a statistically significant relationship with perceived stress level, including stress reduction and problem analysis (as risk factors), while cognitive restructuring (as a protective factor). See Table 6 .
Comparisons between domestic and international students
We compared health anxiety and perceived stress levels of the Hungarian and international students’ groups using the Mann–Whitney test. In the case of health anxiety, the results showed that there were significant differences between the two groups ( W = 149,431; p = 0.038) and international students’ levels were higher. Also, there was a significant difference in the perceived stress level between the two groups ( W = 141,024; p < 0.001), and the international students have increased stress levels compared to the Hungarian ones.
Comparisons between genders within students’ groups (International vs Hungarian)
Firstly, we compared the international men’s and women’s health anxiety and stress levels using the Mann–Whitney test. The results showed that the international women’s health anxiety ( W = 11,810; p = 0.012) and perceived stress ( W = 10,371; p < 0.001) levels were both significantly higher than international men’s values. However, in the Hungarian sample, women’s health anxiety was significantly higher than men’s ( W = 69,643; p < 0.001), but there was no significant difference in perceived stress levels among between Hungarian women and men ( W = 75,644.5; p = 0.064).
Relationship between health anxiety and perceived stress
We correlated the general health anxiety and perceived stress using Spearman’s rank correlation. There was a significant moderate positive relationship between the two variables ( p < 0.001; ρ = 0.446). Within the Hungarian students, there was a significant correlation between health anxiety and perceived stress ( p < 0.001; ρ = 0.433), similarly among international students as well ( p < 0.001; ρ = 0.465).
In our study, we found that individuals who were characterized by a preference for certain coping strategies reported significantly higher perceived stress and/or health anxiety than those who used other coping methods. These correlations can be found in both the Hungarian and international students. In the light of our results, we can say that 48.4% of the international students used wishful thinking as their preferred coping method while around 43% of the Hungarian students used primarily cognitive restructuring to overcome their problems.
Regulation of emotion refers to “the processes whereby individuals monitor, evaluate, and modify their emotions in an effort to control which emotions they have, when they have them, and how they experience and express those emotions” [ 41 ]. There is an overlap between emotion-focused coping and emotion regulation strategies, but there are also differences. The overlap between the two concepts can be noticed in the fact that emotion-focused coping strategies have an emotional regulatory role, and emotion regulation strategies may “tax the individual’s resources” as the emotion-focused coping strategies do [ 23 , 42 ]. However, in emotion-focused coping strategies, non-emotional tools can also be used to achieve non-emotional goals, while emotion regulation strategies may be used for maintaining or reinforcing positive emotions [ 42 ].
Based on the cognitive-behavioral model of health anxiety, emotion-regulating strategies can regulate the physiological, cognitive, and behavioral consequences of a fear response to some degree, even when the person encounters the conditioned stimulus again [ 12 , 43 ]. In the long run, regular use of these dysfunctional emotion control strategies may manifest as functional impairment, which may be associated with anxiety disorders. A detailed study that examined health anxiety in the view of the cognitive-behavioral model found that, regardless of the effect of depression, there are significant and consistent correlations between certain dimensions of health anxiety and dysfunctional coping and emotional regulation strategies [ 12 ].
Similar to our current study, other studies have found that health anxiety was positively correlated with maladaptive emotion regulation and negatively with adaptive emotion regulation [ 44 ], and in the case of state anxiety that emotion-focused coping strategies proved to be less effective in reducing stress, while active coping leads to a sense of subjective well-being [ 17 , 27 , 45 , 46 , 47 ]
SHAI values were found to be high in other studies during the pandemic, and the SHAI results of the international students in our study were found to be even slightly higher compared to those studies [ 44 , 48 ]. Besides, anxiety values for women were found to be higher than for men in several studies [ 44 , 48 , 49 , 50 ]. This was similar to what we found among the international students but not among the Hungarian ones. We can speculate that the ability to contact someone, the closeness of family and beloved ones, familiarity with the living environment, and maybe less online search about the coronavirus news could be factors counting towards that finding among Hungarian students. Also, most international students were enrolled in health-related study programs and his might have affected how they perceived stress/anxiety and their preferred coping strategies as well. Literature found that students of medical disciplines could have obstacles in achieving a healthy coping strategy to deal with stress and anxiety despite their profound medical knowledge compared to non-health-related students [ 51 , 52 ]. Literature also stressed the immense need for training programs to help students of medical disciplines in adopting coping skills and stress-reducing strategies [ 51 ].
The findings of our study may be a starting point for the exploration of the linkage between perceived stress, health anxiety, and coping strategies when people are not in their domestic context. People who are away from their home and friends in a relatively alien environment may tend to use coping mechanisms other than the adequate ones, which in turn can lead to increased levels of perceived stress.
Furthermore, our results seem to support the knowledge that deep-rooted health anxiety is difficult to change because it is closely related to certain coping mechanisms. It was also addressed in the literature that personality traits may have a significant influence on the coping strategy used by a person [ 53 ], revealing sophisticated and challenging links to be considered especially during training programs on effective coping and management skills. On the other hand, perceived stress which has risen significantly above the average level in the current pandemic, can be most effectively targeted by the well-formulated recommendations and advice of major international health organizations if people successfully adhere to them (e.g. physical activity; proper and adequate sleep; healthy eating; avoiding alcohol; meditation; caring for others; relationships maintenance, and using credible information resources about the pandemic, etc.) [ 1 , 54 ]. Furthermore, there may be additional positive effects of these recommendations when published in different languages or languages that are spoken by a wide range of nationalities. Besides, cognitive behavioral therapy techniques, some of which are available online during the current pandemic crisis, can further reduce anxiety. Also, if someone does not feel safe or fear prevails, there are helplines to get in touch with professionals, and this applies to the University of Debrecen in Hungary, and to a certain extent internationally.
Naturally, our study had certain limitations that should be acknowledged and considered. The temporality of events could not be assessed as we employed a cross-sectional study design, that is, we did not have information on the previous conditions of the participants which means that it is possible that some of these conditions existed in the past, while others de facto occurred with COVID-19 crisis. The survey questionnaires were completed by those who felt interested and involved, i.e., a convenience sampling technique was used, this impairs the representativeness of the sample (in terms of sociodemographic variables) and the generalizability of our results. Also, the type of recruitment (including social media) as well as the online nature of the study, probably appealed more to people with an affinity with this kind of instrument. Besides, each questionnaire represented self-reported states; thus, over-reporting or under-reporting could be present. It is also important to note that international students were answering the survey questionnaire in a language that might not have been their mother language. Nevertheless, English fluency is a prerequisite to enroll in a study program at the University of Debrecen for international students. As the options for gender were only male/female in our survey questionnaire, we might have missed the views of students who do not identify themselves according to these gender categories. Also, no data on medical history/current medical status were collected. Lastly, we had to make minor changes to the used scales in the different languages for comparability.
The COVID-19 pandemic crisis has imposed a significant burden on the physical and psychological wellbeing of humans. Crises like the current pandemic can trigger unprecedented emotional and behavioral responses among individuals to adapt or cope with the situation. The elevated perceived stress levels during major life events can be further deepened by disengagement from home and by using inadequate coping strategies. By following and adhering to the international recommendations, adopting proper coping strategies, and equipping oneself with the required coping and stress management skills, the associated high levels of perceived stress and anxiety might be mitigated.
Availability of data and materials
The datasets generated and/or analyzed during the current study are not publicly available due to compliance with institutional guidelines but they are available from the corresponding author (LRK) on a reasonable request.
Abbreviations
Centers for Disease Control and Prevention
Coronavirus Disease 2019
Perceived Stress Scale
Short Health Anxiety Inventory
Middle East Respiratory Syndrome
Severe Acute Respiratory Syndrome
Ways of Coping Questionnaire
World Health Organization
World Health Organization. Advice for the public on COVID-19. [Online]. 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public . Accessed 9 Sep 2020.
National Center for Public Health. Opportunities to reduce contact numbers—Community events in relation to COVID-19 virus infection. [Online]. 2020. https://www.nnk.gov.hu/index.php/koronavirus-tajekoztato/549-opportunities-to-reduce-contact-numbers-community-events-in-relation-to-covid-19-virus-infection . Accessed 20 Sep 2020.
GardaWorld. Crisis24 News Alert. [Online]. 2020. https://www.garda.com/crisis24/news-alerts?search_api_fulltext=&na_countries%5B%5D=1431&field_news_alert_categories=All&field_news_alert_crit=All&items_per_page=20 . Accessed 20 Sep 2020.
Tanne JH, Hayasaki E, Zastrow M, Pulla P, Smith P, Rada AG. Covid-19: how doctors and healthcare systems are tackling coronavirus worldwide. Br Med J. 2020;368:m1090.
Article Google Scholar
Sohrabi C, Alsafi Z, O’Neill N, Khan M, Kerwan A, Al-Jabir A, et al. World Health Organization declares global emergency: A review of the 2019 novel coronavirus (COVID-19). Int J Surg. 2020;76:71–6.
Rosenbaum L. Facing Covid-19 in Italy—ethics, logistics, and therapeutics on the epidemic’s front line. N Engl J Med. 2020;382:1873–5. https://doi.org/10.1056/NEJMp2005492 .
Article PubMed Google Scholar
Wang C, Pan R, Wan X, Tan Y, Xu L, Ho CS, et al. Immediate Psychological Responses and Associated Factors during the Initial Stage of the 2019 Coronavirus Disease (COVID-19) epidemic among the general population in China. Int J Environ Res Public Health. 2020;17:1729.
Akour A, Al-Tammemi AB, Barakat M, Kanj R, Fakhouri HN, Malkawi A, et al. The impact of the COVID-19 pandemic and emergency distance teaching on the psychological status of university teachers: a cross-sectional study in Jordan. Am J Trop Med Hyg. 2020;103:2391–9.
Al-Tammemi AB, Akour A, Alfalah L. Is it just about physical health? An online cross-sectional study exploring the psychological distress among university students in Jordan in the Midst of COVID-19 Pandemic. Front Psychol. 2020;11:562213.
Roddenberry A, Renk K. Locus of control and self-efficacy: potential mediators of stress, illness, and utilization of health services in college students. Child Psychiatry Hum Dev. 2010;41:353–70.
Racic M, Todorovic R, Ivkovic N, Masic S, Joksimovic B, Kulic M. Self- perceived stress in relation to anxiety, depression and health-related quality of life among health professions students: a cross-sectional study from Bosnia and Herzegovina. Slov J Public Heal. 2017;56:251–9.
Görgen SM, Hiller W, Witthöft M. Health anxiety, cognitive coping, and emotion regulation: a latent variable approach. Int J Behav Med. 2014;21:364–74.
Abramowitz JS, Braddock A. Psychological treatment of health anxiety and hypochondriasis: a biopsychosocial approach. Boston: Hogrefe Publishing; 2008.
Google Scholar
Taylor S, Asmundson GJG. Treating health anxiety: a cognitive-behavioral approach. 1st ed. New York: Guilford Press; 2004.
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders,(DSM-5). 5th edition. Washington, DC: American Psychiatric Association; 2013.
Wheaton MG, Abramowitz JS, Berman NC, Fabricant LE, Olatunji BO. Psychological predictors of anxiety in response to the H1N1 (swine flu) pandemic. Cognit Ther Res. 2012;36:210–8.
Asmundson GJG, Taylor S. How health anxiety influences responses to viral outbreaks like COVID-19: What all decision-makers, health authorities, and health care professionals need to know. J Anxiety Disord. 2020;71:102211.
Taha SA, Matheson K, Anisman H. The 2009 H1N1 influenza pandemic: the role of threat, coping, and media trust on vaccination intentions in Canada. J Health Commun. 2013;18:278–90.
Phua DH, Tang HK, Tham KY. Coping responses of emergency physicians and nurses to the 2003 severe acute respiratory syndrome outbreak. Acad Emerg. 2005;12:322–8.
Teasdale E, Yardley L, Schlotz W, Michie S. The importance of coping appraisal in behavioural responses to pandemic flu. Br J Health Psychol. 2012;17:44–59.
Sim K, Huak Chan Y, Chong PN, Chua HC, Wen SS. Psychosocial and coping responses within the community health care setting towards a national outbreak of an infectious disease. J Psychosom Res. 2010;68:195–202.
Lazarus RS. Stress and emotion: a new synthesis. London: Free Association Books; 1999.
Lazarus RS, Folkman S. Stress, appraisal, and coping. New York: Springer; 1984.
Lazarus RS, Folkman S. Transactional theory and research on emotions and coping. Eur J Pers. 1987;1:141–69.
Cai H, Tu B, Ma J, Chen L, Fu L, Jiang Y, et al. psychological impact and coping strategies of frontline medical staff in hunan between January and March 2020 during the Outbreak of Coronavirus Disease 2019 (COVID-19) in Hubei. China Med Sci Monit. 2020;26:e924171.
PubMed Google Scholar
Chua SE, Cheung V, Cheung C, McAlonan GM, Wong JWS, Cheung EPT, et al. Psychological effects of the SARS outbreak in Hong Kong on high-risk health care workers. Can J Psychiatry. 2004;49:391–3. https://doi.org/10.1177/070674370404900609 .
Flesia L, Monaro M, Mazza C, Fietta V, Colicino E, Segatto B, et al. Predicting perceived stress related to the Covid-19 outbreak through stable psychological traits and machine learning models. J Clin Med. 2020;9:3350.
Khalid I, Khalid TJ, Qabajah MR, Barnard AG, Qushmaq IA. Healthcare workers emotions, perceived stressors and coping strategies during a MERS-CoV outbreak. Clin Med Res. 2016;14:7–14.
Gee S, Skovdal M. The role of risk perception in willingness to respond to the 2014–2016 West African Ebola outbreak: a qualitative study of international health care workers. Glob Heal Res Policy. 2017;2:21.
Gerhold L. COVID-19: Risk perception and Coping strategies. Results from a survey in Germany. PsyArXiv Prepr. 2020.
Auerbach RP, Alonso J, Axinn WG, Cuijpers P, Ebert DD, Green JG, et al. Mental disorders among college students in the World Health Organization World Mental Health Surveys. Psychol Med. 2016;46:2955–70.
Bíró É. Studies on the mental health of students in higher education. University of Debrecen; 2014. https://dea.lib.unideb.hu/dea/handle/2437/195979 . Accessed 15 Feb 2021.
The University of Debrecen. Facts and Figures. [Online]. 2020. https://www.edu.unideb.hu/page.php?id=28 . Accessed 25 Dec 2020.
Cohen S. Perceived stress in a probability sample of the United States. In: The social psychology of health. Thousand Oaks: Sage Publications, Inc; 1988. p. 31–67.
Strauder A, Thege BK. Az Észlelt Stressz Kérdőív (PSS) Magyar Verziójának Jellemzői. Mentálhigiéné És Pszichoszomatika. 2006;7:203–16.
Sørlie T, Sexton HC. The factor structure of “The Ways of Coping Questionnaire” and the process of coping in surgical patients. Pers Individ Dif. 2001;30:961–75.
Rózsa S, Purebl G, Susánszky É, Kő N, Szádóczky E, Réthelyi J, et al. Dimensions of coping: Hungarian adaptation of the Ways of Coping Questionnaire. Mentálhigiéné És Pszichoszomatika. 2008; 217–241.
Salkovskis PM, Rimes KA, Warwick HMC. The Health Anxiety Inventory: development and validation of scales for the measurement of health anxiety and hypochondriasis. Psychol Med. 2002;32:843.
Alberts NM, Hadjistavropoulos HD, Jones SL, Sharpe D. The Short Health Anxiety Inventory: a systematic review and meta-analysis. J Anxiety Disord. 2013;27:68–78.
Köteles F, Simor P, Bárdos G. Validation and psychometric evaluation of the Hungarian version of the Short Health Anxiety Inventory (SHAI). Mentálhigiéné és Pszichoszomatika. 2011;12:191–213.
Artino AR. Regulation of Emotion. In: Encyclopedia of Child Behavior and Development. Boston, MA: Springer US; 2011. p. 1236–8.
Gross JJ. The emerging field of emotion regulation: an integrative review. Rev Gen Psychol. 1998;2:271–99.
Kaczkurkin AN, Foa EB. Cognitive-behavioral therapy for anxiety disorders: an update on the empirical evidence. Dialogues Clin Neurosci. 2015;17:337–46.
Jungmann SM, Witthöft M. Health anxiety, cyberchondria, and coping in the current COVID-19 pandemic: Which factors are related to coronavirus anxiety? J Anxiety Disord. 2020;73:102239.
Taha S, Matheson K, Cronin T, Anisman H. Intolerance of uncertainty, appraisals, coping, and anxiety: the case of the 2009 H1N1 pandemic. Br J Health Psychol. 2014;19:592–605.
Umucu E, Lee B. Examining the impact of COVID-19 on stress and coping strategies in individuals with disabilities and chronic conditions. Rehabil Psychol. 2020;65:193–8.
Main A, Zhou Q, Ma Y, Luecken LJ, Liu X. Relations of SARS-related stressors and coping to Chinese college students’ psychological adjustment during the 2003 Beijing SARS epidemic. J Couns Psychol. 2011;58:410–23.
Özdin S, Bayrak ÖŞ. Levels and predictors of anxiety, depression and health anxiety during COVID-19 pandemic in Turkish society: the importance of gender. Int J Soc Psychiatry. 2020;66:504–11.
Taylor S, Landry CA, Paluszek MM, Fergus TA, McKay D, Asmundson GJG. COVID stress syndrome: concept, structure, and correlates. Depress Anxiety. 2020;37:706–14.
Gamonal Limcaoco RS, Mateos EM, Fernández JM, Roncero C. Anxiety, worry and perceived stress in the world due to the COVID-19 pandemic, March 2020. Preliminary results. MedRxiv Prepr. 2020.
Abouammoh N, Irfan F, AlFaris E. Stress coping strategies among medical students and trainees in Saudi Arabia: a qualitative study. BMC Med Educ. 2020;20:124.
Gade S, Chari S, Gupta M. Perceived stress among medical students: to identify its sources and coping strategies. Arch Med Health Sci. 2014;2:80–6.
Leszko M, Iwański R, Jarzębińska A. The relationship between personality traits and coping styles among first-time and recurrent prisoners in Poland. Front Psychol. 2020;10:2969.
Centers for Disease Control and Prevention. Mental Health and Coping During COVID-19 Pandemic. [Online]. 2020. https://www.cdc.gov/coronavirus/2019-ncov/daily-life-coping/managing-stress-anxiety.html . Accessed 9 Sep 2020.
Download references
Acknowledgments
We would like to provide our extreme thanks and appreciation to all students who participated in our study. ABA is currently supported by the Tempus Public Foundation’s scholarship at the University of Debrecen.
This research project did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and affiliations.
Doctoral School of Health Sciences, University of Debrecen, Debrecen, Hungary
Szabolcs Garbóczy, Szilvia Harsányi, Ala’a B. Al-Tammemi & László Róbert Kolozsvári
Department of Psychiatry, Faculty of Medicine, University of Debrecen, Debrecen, Hungary
Szabolcs Garbóczy
Department of Personality and Clinical Psychology, Institute of Psychology, University of Debrecen, Debrecen, Hungary
Anita Szemán-Nagy
Faculty of Medicine, University of Debrecen, Debrecen, Hungary
Mohamed S. Ahmad & Viktor Rekenyi
Department of Social and Work Psychology, Institute of Psychology, University of Debrecen, Debrecen, Hungary
Dorottya Ocsenás
Doctoral School of Human Sciences, University of Debrecen, Debrecen, Hungary
Department of Family and Occupational Medicine, Faculty of Medicine, University of Debrecen, Móricz Zs. krt. 22, Debrecen, 4032, Hungary
Ala’a B. Al-Tammemi & László Róbert Kolozsvári
You can also search for this author in PubMed Google Scholar
Contributions
All authors SG, ASN, MSA, SH, DO, VR, ABA, and LRK have worked on the study design, text writing, revising, and editing of the manuscript. DO, SG, and VR have done data management and extraction, data analysis. Drafting and interpretation of the manuscript were made in close collaboration by all authors SG, ASN, MSA, SH, DO, VR, ABA, and LRK. All authors read and approved the final manuscript.
Corresponding author
Correspondence to László Róbert Kolozsvári .
Ethics declarations
Ethics approval and consent to participate.
Ethical permission was obtained from the Hungarian Ethical Review Committee for Research in Psychology (Reference number: 2020-45). All methods were carried out following the institutional guidelines and conforming to the ethical standards of the declaration of Helsinki. All participants were informed about the study and written informed consent was obtained before completing the survey.
Consent for publication
Not Applicable.

Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Garbóczy, S., Szemán-Nagy, A., Ahmad, M.S. et al. Health anxiety, perceived stress, and coping styles in the shadow of the COVID-19. BMC Psychol 9 , 53 (2021). https://doi.org/10.1186/s40359-021-00560-3
Download citation
Received : 07 January 2021
Accepted : 26 March 2021
Published : 06 April 2021
DOI : https://doi.org/10.1186/s40359-021-00560-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Health anxiety
- Perceived stress
- Coping styles
- University students
BMC Psychology
ISSN: 2050-7283
- General enquiries: [email protected]
- Open access
- Published: 29 March 2024
Sociodemographic disadvantage in the burden of stress and academic performance in medical school: implications for diversity in medicine
- Danielle Eames ORCID: orcid.org/0000-0003-3357-6658 1 ,
- Shelby Thomas ORCID: orcid.org/0009-0009-2246-837X 1 ,
- Kaden Norman ORCID: orcid.org/0009-0001-1958-9391 1 ,
- Edward Simanton 1 &
- Anne Weisman ORCID: orcid.org/0000-0002-5121-0177 1
BMC Medical Education volume 24 , Article number: 348 ( 2024 ) Cite this article
Metrics details
Nontraditional students bring to medicine inherent characteristics and perspectives that enrich the learning environment and contribute to expanding diversity in medicine. However, research has shown that these students, by virtue of their sociodemographic backgrounds, face unique challenges in medical education, which ultimately place them at a disadvantage compared to their peers. The purpose of this study is to explore relationships between sociodemographic characteristics, stress, and academic performance, in the context of outcomes that may be undermining efforts to diversify the physician workforce.
Using a retrospective observational cohort methodology, we examined institutional and USMLE exam performance data in conjunction with Perceived Stress Scale-4 survey results from six cohorts of students at Kirk Kerkorian School of Medicine at UNLV ( n = 358). Using independent samples t-test, mean stress and academic performance were compared between four sociodemographic groups: first-generation college students, underrepresented in medicine (URM), socioeconomically disadvantaged, and age 30 + at matriculation. Results were considered significant where P ≤ .05.
First-generation college students had significantly higher stress at the end of third year clerkships (mean 7.8 vs. 6.8, P * = .03). URM students had significantly lower scores on preclinical exams (mean 81.37 vs. 83.07, P * = .02). The students who were age 30 + at matriculation had significantly lower exam scores on all academic performance measures.
Our results echo historic trends in academic performance for racial and ethnic minority students, and we present recent evidence of academic performance disparities based on age at matriculation. Residency program directors continue to use test scores as a primary metric to screen applicants and thus, poor academic performance has profound consequences on career trajectory. Finally, significantly higher stress in the first-generation students may be evidence of underlying psychological distress. Expanding the sociodemographic diversity among physicians, and by extension, medical students, has long been recognized as fundamental to addressing inequities in healthcare. However, results from our study suggest that aspects of medical education are unfavorable and disadvantageous for first-generation, URM, and older medical students. A deeper understanding of the interplay between sociodemographic characteristics and success in medical school is paramount as we pursue diversity in medicine.
Peer Review reports
Research has shown that being a medical student is associated with a greater degree of perceived stress, reduced overall wellbeing [ 1 , 2 , 3 ], higher rates of depression, anxiety, and burnout [ 4 , 5 , 6 , 7 ] and that these experiences can negatively impact academic performance [ 1 , 2 , 3 , 8 ]. The 2022 National College Health Assessment (NCHA) survey, which collects data on factors that students perceive to affect their academic performance, reported that the top three factors cited by graduate and professional students were stress (33%), anxiety (28%), and depression (20%) [ 8 ]. Common sources of stress in medical education include assessment-related performance pressure, excessive workload, conflicts in school-life balance and personal relationships, peer relations, health concerns, the learning environment, and administrative failures [ 6 , 7 , 9 , 10 , 11 ]. In addition to these stressors faced by all medical students, nontraditional students Footnote 1 face additional stressors that further compromise health and well-being, detract from academic success, and diminish sense of fulfillment with medical training [ 1 , 2 , 3 , 10 , 12 , 13 , 14 , 15 ].
In a study of 69,722 students from more than 100 different U.S. post-secondary institutions, Stevens, et. al. (2018), found that discrimination is a common stressor for racial/ethnic minority undergraduate students, and that these experiences negatively impact their academic performance [ 16 ]. These students are also vulnerable to “minority status stress,” which refers to heightened feelings of not belonging that interfere with social integration [ 17 ]. Research on older medical students, though sparse, suggests that these students experience a greater overall stress burden due to additional responsibilities outside of medical school and that these responsibilities interfere with studying [ 18 ]. Mason et. al. (2018) looked at several indicators of well-being in first-generation medical students. They found significant negative correlations between perceived stress and quality of life across physical, psychological, social, and environmental domains [ 15 ]. Studies have shown that financial stress related to excess education debt disproportionately affects racial and ethnic minority, low-income, and first-generation college students [ 13 , 19 ]. Furthermore, studies show that the accumulation of large amounts of debt during medical school is associated with increased stress [ 1 , 13 ], poorer academic performance [ 1 ], increased risk for burnout [ 5 ], and pursuit of higher-paying subspecialties [ 1 , 20 ].
Nontraditional students bring to medicine inherent characteristics and perspectives that enrich the learning environment [ 21 ] and contribute to expanding sociodemographic diversity among physicians [ 22 , 23 , 24 ]. However, by virtue of their sociodemographic backgrounds, these students face unique challenges in medical education, which ultimately places them at a disadvantage compared to their peers. The purpose of this study is to explore relationships between sociodemographic characteristics, stress, and academic performance, in the context of outcomes that may be undermining efforts to diversify the physician workforce. Utilizing data collected at Kirk Kerkorian School of Medicine at UNLV, we evaluated stress and academic performance in four categories of nontraditional students with the hypothesis that, when compared to their counterparts, these students would have higher perceived stress, lower academic performance, and possibly both. This study will contribute to the growing body of knowledge on stress and academic performance in medical education for those who approach medical school from a place of sociodemographic disadvantage. To the best of our knowledge, this study is among the first to look at stress, at multiple predetermined points in the curriculum, juxtaposed with academic performance. Notably, we report findings on “older” students, a small but richly diverse subset of nontraditional medical students on which research is considerably lacking.
Sociodemographic characteristics, Perceived Stress Scale-4 (PSS) scores, and exam performance data on 358 of 360 students who matriculated to the Kirk Kerkorian School of Medicine as part of the graduating classes of 2021 through 2026 were utilized for the purposes of this retrospective observational cohort study. Due to substantial fluctuations in graduation timelines, 2 students were not included in any of the data analyses. All study participant data were deidentified prior to retrieval and utilized in accordance with existing IRB-approved protocols. The sociodemographic groups, data, and respective analyses are described below.
Sociodemographic group classification and justification
Selection of nontraditional sociodemographic groups.
Using data collected at the time of admission, students were sorted into one or more of the following demographic groups: First-Generation College Student (FGCS), Underrepresented in Medicine (URM), Socioeconomically Disadvantaged (SED), and Age 30 years or older (Age 30 +) at matriculation. These groups were chosen based on data published by the Association of American Medical Colleges (AAMC) showing that these groups are currently underrepresented among medical students. Nationally, of the medical school matriculants in 2022, 11.2% were first-generation college students [ 25 ], 22.7% were URM [ 26 ], 21.5% were socioeconomically disadvantaged [ 27 ], and 5.7% were 29 years of age or older [ 28 ]. Furthermore, a 2017 analysis of socioeconomic diversity among US medical students found that, in 2017, 24% of matriculants reported parental income in the top 5% (greater than $225,251) of all US households and over half were from households in the top 20% (greater than $121,019), findings that have been consistent for the past 30 years [ 29 ]. Based on these data, we considered these categories (e.g., FGCS, URM, SED, and Age 30 +) to be appropriate nontraditional sociodemographic groups to be included in our study.
First-Generation College Students: FGCS vs. CGCS
Following the AAMC definition, students whose “most highly educated parent/guardian has up to the equivalent of some college but earned no degree” were included in the “FGCS” group. These students were compared to continuing-generation college students (“CGCS”) [ 25 ].
Underrepresented in Medicine: URM vs. non-URM
The AAMC defines underrepresented in medicine (URM) as “racial and ethnic populations that are under-represented in the medical profession relative to their numbers in the general population" [ 30 ]. This definition is purposefully vague with regards to race and ethnicity to allow for geographic differences and/or temporal changes in population diversity. Students who self-selected “Black/African American”, “Hispanic/Latinx”, and “Native American” (e.g., American Indian, Hawaiian Native, or Alaskan Native) were included in the “URM” group; all other race and ethnic groups were included in the “non-URM group”.
Socioeconomic Disadvantage: SED vs. non-SED
Socioeconomic advantage or disadvantage is identified in medical school applicants by combining conventional socioeconomic status (SES) metrics (e.g., annual household income) with additional information regarding parental education and occupation (EO); together, these metrics are known as SES-EO categories, and are stratified into quintiles [ 29 ]. Students from households with an annual income below $45,600, or whose parents have “less than a bachelor’s degree, or parents with any degree and a service, clerical, skilled, or unskilled occupation” are classified as SES-EO 1 or 2 and are considered SES-EO disadvantaged. Students from households with annual income above $45,601 or with at least 1 parent with “a bachelor’s degree or higher, and an executive, managerial, or professional occupation” are grouped into EO-3, EO-4, or EO-5 [ 29 ]. Our study group “SED” includes SES-EO 1 and 2 students. Students in SES-EO 3, 4, and 5 categories served as the comparison group “non-SED”.
Age at matriculation: age 30 + vs. under 30
To look at differences based on age, we compared students who were 30 years of age or older (“age 30 + ”) to those who were 29 years or younger (“under 30”) at the time of matriculation to medical school. Data from the 2022 AAMC Matriculating Student Questionnaire (MSQ) showed that the vast majority of matriculants were 25 years of age or less (82.9%) [ 28 ]. Using this as a reference point, we deemed 30 years of age to be sufficiently different from the average, and thus an appropriate cutoff to represent an “older” medical student.
Stress data
Perceived stress scale-4.
As part of continuous internal quality improvement efforts, institutional program evaluation data has been collected from all students beginning in 2017 with the matriculation of the inaugural class at Kirk Kerkorian School of Medicine at UNLV. Part of this evaluation includes assessing student stress using the Short Form Perceived Stress Scale Questionnaire (PSS-4), a widely used tool to quantify perceived general stress [ 28 , 31 , 32 , 33 ]. The PSS-4 consists of four questions designed to measure perceived stress over the previous month (see Appendix 1 for an outline of the survey). Each item is scored on a scale of 0 (very low stress) to 4 (very high stress), and the cumulative score out of 16 correlates with the degree of perceived stress [ 31 , 32 ]. PSS-4 surveys are collected at four educational milestones: (1) prior to matriculation (“pre-matriculation”), (2) at the end of the preclinical phase, (3) at the end of third-year clerkship rotations, and (4) immediately before students participate in the residency matching program (“pre-match”) . Due to the rolling nature of program evaluation data collection, all students had not participated in data collection at all educational milestones at the time of the study. For example, all 358 students included in the study had completed the pre-matriculation survey, while only 139 students had completed the pre-match survey.
Academic performance data
Institutional nbme exam performance: “preclinical exam average” and "clinical subject exam average”.
In the preclinical, organ-systems-based curriculum, student knowledge is assessed using the Customized Assessment Service from the National Board of Medical Examiners (NBME). Each organ-system block varies slightly in length, and, due to evolving curricular structure, each cohort of students have taken a different number of preclinical assessments. For the purposes of comparing academic performance, an average exam score was calculated for each study participant using their scores on all administered preclinical exams. This average exam score for each study participant was then used in the statistical analysis. The results of this analysis are reported as “preclinical exam average”.
Clinical knowledge is assessed during the third year of medical school using Clinical Subject NBME Exams, covering six core specialties (e.g., Internal Medicine, Family Medicine, Pediatrics, Obstetrics and Gynecology, Surgery, and Psychiatry). As described for the preclinical exams, the average exam score calculated for each study participant was used in the statistical analysis. The results of this analysis are reported as “clinical subject exam average”.
USMLE performance: “Step 1” and “Step 2 CK”
Three-digit numeric scores on the Step 1 and Step 2 CK United States Medical Licensing Examinations (USMLE) for each study participant were used in the statistical analysis. Students sit for Step 1 after the preclinical phase and must pass the exam before being promoted to the clinical phase of the curriculum. Students may sit for Step 2 CK at any time after Step 1. Therefore, at the time of this study, some students may have taken none, one, or both USMLE exams. Additionally, we did not include students who took the USMLE Step 1 after January 26, 2022, when the exam moved to Pass/Fail score reporting. Results of this statistical analysis are reported as “Step 1 average” and “Step 2 CK average”.
Statistical analysis
Using these preexisting data sets, independent samples t-tests were calculated with Statistical Package for Social Sciences (SPSS) (version 27) software to assess for differences in PSS-4 scores and academic performance between FGCS vs. CGCS; URM vs. non-URM; SED vs. non-SED; and Age 30 + vs. Under 30. Results were considered significant (indicated as P* ) for two-sided P values where P ≤ 0.05. All P values are reported with equal variances assumed unless otherwise noted.
Of the 358 students included in the study, nearly all of them (97.2%) fall into at least one of our nontraditional sociodemographic groups (Table 1 ).
FGCS vs. continuing-generation college students (CGCS)
Compared to their CGCS peers, we found that FGCS had lower stress at the first two educational milestones (e.g., pre-matriculation and the end of the preclinical phase), roughly the same stress at the final educational milestone (e.g., as students approached the residency match), but, between these points, at the end of third-year clerkships, stress among the FGCS was significantly higher than that of their CGCS counterparts (mean 7.8 vs. 6.8; 95% CI [0.09 to 1.98], P* = 0.03) (Fig. 1 ; see also Supplementary Table 1, Appendix 2 ). While the FGCS and CGCS performed roughly the same on the preclinical institutional exams, the FGCS had lower average scores on all other academic performance measures. However, none of these differences met statistical significance (Table 2 ).
Results of mean PSS-4 score comparison between FGCS vs. CGCS. While actual scores can range from 0 to 16, the y-axis has been amended to a range of 1 to 10 in order to visually present the data. Abbreviations : FGCS first-generation college student, CGCS continuing-generation college student, PSS-4 Perceived Stress Scale-4
Underrepresented in Medicine (URM) vs. non-URM
The comparison of stress between URM and non-URM students showed that URM students had lower stress at each educational milestone until the last PSS-4 survey collection, immediately before students participate in the residency match (“pre-match”). However, none of the differences in stress between URM and non-URM students met statistical significance (Table 3 ). When we compared academic performance, we found that URM students had lower average exam scores on institutional and USMLE exams; however, the only statistically significant difference was performance on the preclinical NBME exams (mean 81.37 vs. 83.07; 95% CI [-3.17 to -0.23], P* = 0.02) (Figs. 2 and 3 ; see also Supplementary Table 2, Appendix 2 ).
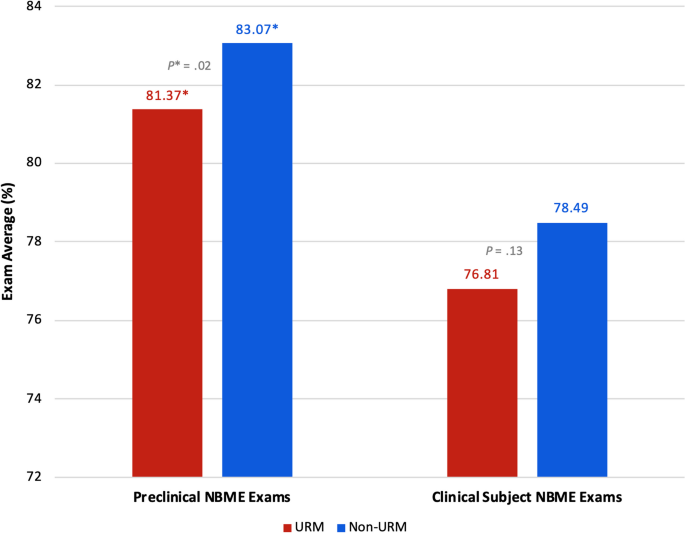
Results of institutional preclinical and clinical subject NBME exam performance between URM vs. non-URM students. While actual scores can range from 0 to 100, the y-axis has been amended to a range of 72 to 84 in order to visually present the data. Abbreviations : URM underrepresented in medicine, NBME National Board of Medical Examiners
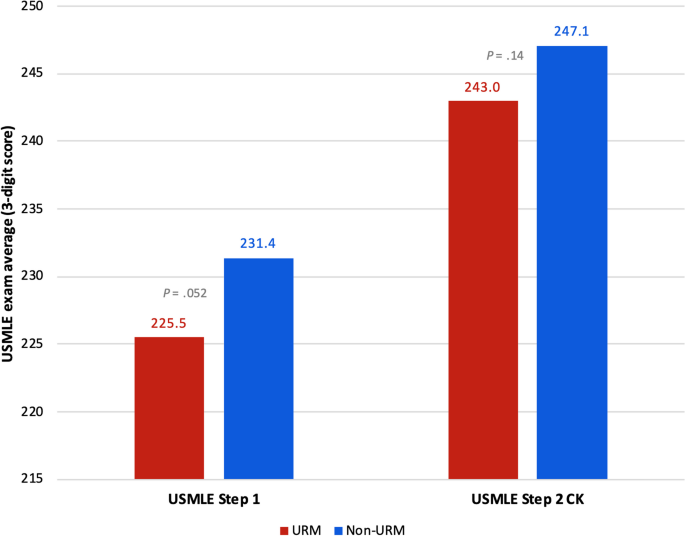
Results of USMLE performance between URM vs. non-URM students. While actual scores can range from 0 to 300, the y-axis has been amended to a range of 215 to 250 in order to visually present the data. Abbreviations : URM underrepresented in medicine, USMLE United States Medical Licensing Examination
Socioeconomic Disadvantage (SED) vs. non-SED
Socioeconomically disadvantaged (SED) students had higher stress at the first three educational milestones (e.g., pre-matriculation, the end of the preclinical phase, and the end of third-year clerkships) (Table 4 ), and lower average performance on the clinical subject and USMLE Step 1 exams (Table 5 ) compared to their non-SED counterparts. However, these differences were not statistically significant.
Age 30 + at matriculation vs. under 30
The comparison of stress between the Age 30 + and Under 30 students revealed lower stress among the Age 30 + cohort at the first three educational milestones, and higher stress only before the residency match (Table 6 ); however, none of these differences were statistically significant. When we compared academic performance between these two groups, the Age 30 + students had significantly lower average exam scores across all academic performance measures (Figs. 4 and 5 ; see also Supplementary Table 3, Appendix 2 ). Results of institutional exam performance are shown in Fig. 4 , with students who were Age 30 + at matriculation scoring significantly lower on the preclinical exams (mean 80.48 vs. 82.95; 95% CI [-4.52 to -0.42]; P* = 0.02) and the clinical subject exams (mean 74.01 vs. 78.66; 95% CI [-7.39 to -1.92]; P* < 0.001). When we looked at USMLE performance (Fig. 5 ), we again found that students who were Age 30 + at matriculation scored significantly lower on both Step 1 (mean 220.8 vs. 231.4; 95% CI [-18.07 to -3.17]; P* = 0.005) and Step 2 CK (mean 234.9^ vs. 247.6^; 95% CI [-22.11 to -3.30]; P^ = 0.01, equal variance NOT assumed). Of note, the statistical analysis of USMLE Step 2 CK performance in this group violated the assumption of equal variances. Nevertheless, the results are included in the figure for transparency and visual continuity.
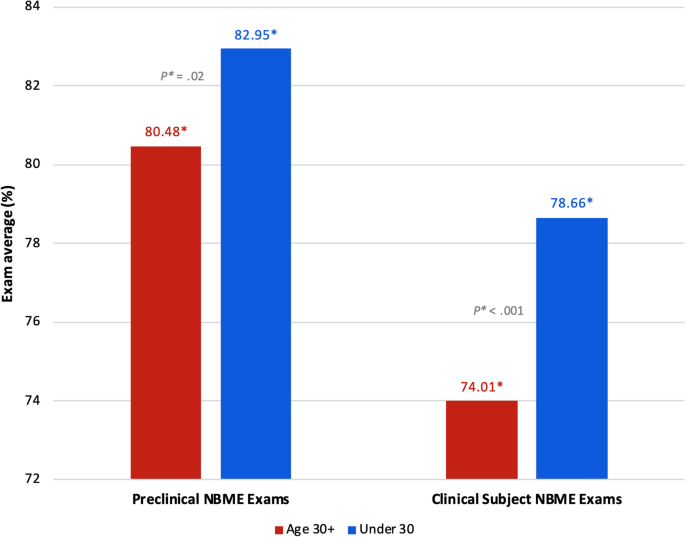
Results of institutional NBME exam performance between students age 30 + vs. under 30 at matriculation. While actual scores can range from 0 to 100, the y-axis has been amended to a range of 72 to 84 in order to visually present the data. Abbreviations : NBME National Board of Medical Examiners
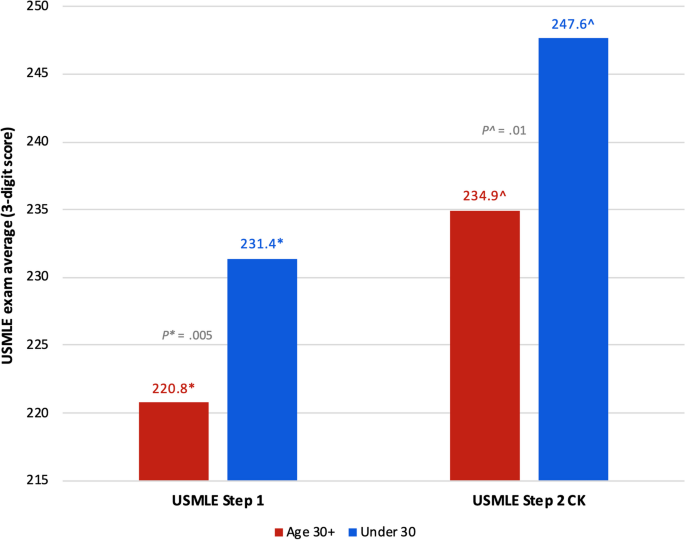
Results of USMLE performance between students age 30 + vs. under 30 at matriculation. While actual scores can range from 0 to 300, the y-axis has been amended to a range of 215 to 250 in order to visually present the data. Abbreviations : USMLE United States Medical Licensing Examination ^equal variances NOT assumed
- Academic performance
URM students scored lower, on average, than their non-URM peers on all academic performance measures, with statistically significant lower average performance on the preclinical exams (Figs. 2 and 3 ; see also Supplementary Table 2, Appendix 2 ). Although these results were only partially statistically significant, this trend in academic performance among URM students is important because it echoes historic trends in assessment performance disparities impacting racial and ethnic minority students [ 34 , 35 , 36 , 37 , 38 ]. Research on the achievement gap in medical education has highlighted how structural inequities in education, stemming from poorly funded K-12 schools that primarily serve low-income and minority children, lead to disparities in performance on standardized exams, including the Medical College Admission Test (MCAT) and United States Medical Licensing Examinations (USMLE) [ 34 , 35 , 36 ]. The most recent AAMC report on medical school applicants and matriculants, which included data from the 2022–2023 application cycle, showed that URM students (Black/African American, Hispanic/Latino/Spanish, and American Indian/Alaskan Native/Native Hawaiian/Pacific Islander) had lower average GPAs and MCAT scores than non-URM students [ 39 ]. A 2019 study led by a team at the National Board of Medical Examiners (NBME), found that Hispanic and Black students were significantly more likely to score lower on any of the Step exams compared to White students, and that nearly half of all students who initially fail Step 1 are racial/ethnic minority students [ 37 ].
Students who were 30 years of age or older at matriculation had significantly lower average exam scores across all academic performance measures compared to their younger counterparts (Figs. 4 and 5 ; see also Supplementary Table 3, Appendix 2 ). These findings indicate that there is a need that is not being met for these students. Previous research, though considerably outdated, has suggested that older medical students may have different learning strategies than their younger counterparts. In a 1998 study at McGill University, Feil et. al. found that older medical students approached learning more abstractly, with concern for the thought processes involved in basic and clinical science, while younger students were more inclined to study by memorizing facts for a test for the sake of getting good grades [ 40 ]. In 2000, Kick et. al. reported that older students perceived medical school to be more intrusive upon their deeply developed personal lives, and that responsibilities at home made it difficult to study [ 18 ]. Given the paucity of recent research on the experiences of older medical students, our findings contribute immensely to the literature. Older medical students bring valuable life experience to patient care and further research to identify factors that may be undermining academic performance among these students is necessary.
Surprisingly, the only comparison of stress that showed statistically significant higher stress among any of our study groups was between first-generation and continuing-generation college students at the end of third-year clerkships (Fig. 1 ; see also Supplementary Table 1, Appendix 2 ). We posit that, because we found evidence of significantly higher perceived stress among only first-generation college students and specifically at the end of third year clerkships, a time when FGCS are exposed to a variety of unfamiliar clinical settings and a highly competitive learning environment, this unique finding may be related to imposter phenomenon.
Imposter phenomenon is characterized by an overwhelming belief that one does not belong in a certain setting despite evidence to the contrary and fear about being discovered as a “fraud” [ 41 ]. People experiencing imposter phenomenon have chronic self-doubt and are unable to internalize personal achievements [ 41 ]. Levant, et. al. (2020) found significant correlations between stress (measured with the 10-item perceived stress scale [PSS-10]) and imposter feelings (measured with the Clance Impostor Phenomenon Scale), and that PSS-10 scores were 28–31% higher in those experiencing imposter phenomenon [ 42 ]. Studies on FGCS at the undergraduate level have shown that these students more frequently report difficulty fitting into campus culture and often doubt their abilities to succeed, feelings that are directly related to imposter syndrome [ 43 , 44 ]. Canning, et. al. (2019) looked at associations between peer competition, generational status, and imposter feelings in students taking Science, Technology, Engineering, and Mathematics (STEM) undergraduate courses [ 45 ]. They found that FGCS were significantly more likely to experience imposter feelings in settings where perceived competition was increased (e.g., STEM courses) [ 45 ]. Although further investigation is needed to make a definitive conclusion about the source of the increased stress in this study group, our results parallel that of previous research on the experience of imposter phenomenon among first-generation college students. This theory is further supported by the absence of significant reductions in exam performance among the FGCS in our study, as this provides evidence that these students are not academically inferior to their peers.
Negative findings
In none of the study groups did we find both higher stress and lower academic performance. In fact, our results show that URM and Age 30 + students arrived at medical school with the lowest reported stress of any group (Tables 3 and 6 , respectively). Based on socioeconomic disadvantage (SED), no significant differences in stress or academic performance were found. Our lack of any significant differences among SED students compared to their non-SED counterparts, particularly with regards to stress, was surprising, as the association of debt and stress is well-established and existing literature has shown increased stress in these students [ 1 , 13 ]. Given the sociodemographic diversity of our study participants (Table 1 ), it would be reasonable to infer that a culture of inclusion and acceptance exists among the students, which may be protective against stress and its sequela.
Additionally, these findings may reflect individual differences in stress appraisal and resilience. Research on stress theory holds that the effects of stress depend, at least in part, on whether the stress is perceived as enhancing or debilitating [ 46 ]. For those who perceive stress as enhancing, it can improve performance and enhance motivation to overcome a challenge [ 46 ]. Perhaps the FGCS in our study, while they have significantly higher stress than CGCS at the end of third-year clerkships, may be less inclined to appraise stress as a negative factor, and thus academic performance was not impacted. The older students significantly under-performed on all academic measures (Figs. 4 and 5 ) yet report some of the lowest stress levels of anyone until they approach the residency match (Table 6 ). Studies on older medical students indicate that they are more likely to hold an internal locus of control, demonstrate greater critical thinking abilities, and have an increased propensity for self-reflection [ 47 , 48 ]. Having led full lives, with prior careers and other life experience, it is possible the older students approach the burden of medical school differently and may be more accepting of their personal limitations. Moreover, by virtue of their nontraditional sociodemographic backgrounds, these students may possess greater resilience. If our nontraditional students have lived experiences overcoming greater adversity, then they may be less inclined to perceive, or report, increases in stress.
Limitations
Our study has several limitations. Our unusually diverse but small study population involving students from a single institution may limit the generalizability of our results. It is likely that our small sample size contributes to many of our comparisons being found statistically non-significant. Our statistical analysis does not account for students who fall into multiple sociodemographic groups. The collection of program evaluation data by Kirk Kerkorian School of Medicine necessitates that the surveys be a required component of the curriculum. Because of this, it is possible that our results were confounded by individual differences in attention and reflection on the survey questions. While the PSS-4 was designed to be better suited for settings in which respondents may not have the time or desire to complete the longer versions of the PSS (e.g., the 10-item and 14-item questionnaires) [ 31 ], and indeed this was the rationale for using the Short Form PSS, it is possible for students to simply click though the survey because it is required to do so without responding thoughtfully to the survey questions. If this is the case, then the survey results would not reflect what we are trying to measure. Lastly, the statistical analysis of differences in USMLE Step 2 CK performance in the “age 30 + ” group violated the assumption of equal variances which remains unexplained by our data set.
Implications
Expanding the sociodemographic diversity among physicians, and by extension, medical students, has long been recognized as fundamental to addressing healthcare inequities in the US [ 49 ]. In 2009, the Liaison Committee on Medical Education (LCME) began implementing accreditation standards regarding the benefits of diversity, which now also include policies on anti-discrimination, cultural competency, and addressing disparities in social determinants of health [ 50 , 51 , 52 ]. Today, considerable effort is put towards increasing the matriculation of students from nontraditional sociodemographic backgrounds. Medical schools have universally adopted holistic admissions policies that recognize socioeconomic status, demographic characteristics, and life experiences of applicants to encourage the matriculation of nontraditional students [ 24 , 53 , 54 , 55 ]. Pipeline programs have been implemented in some areas to recruit students to medicine from community colleges, where many nontraditional students begin their post-secondary education [ 22 , 23 , 24 , 36 , 56 , 57 ]. Despite these efforts, however, disparities in the availability and quality of healthcare resources, burden of cost, health insurance coverage, patient outcomes, general health status, and overall life expectancy continue to exist for racial/ethnic minority, low-income, and inner city, and rural communities [ 58 ].
The purpose of this study was to explore relationships between sociodemographic characteristics, stress, and academic performance, in the context of outcomes that may be undermining efforts to diversify the physician workforce. Nontraditional students bring to medicine inherent characteristics and perspectives that enrich the learning environment [ 21 ], and contribute to expanding access to culturally competent, linguistically appropriate healthcare services for underserved communities in the US [ 22 , 23 , 24 ]. With a projected shortage of up to 124,000 physicians by 2034 [ 59 ], and the significant lag time between embarking on post-secondary education and independent practice, increasing the diversity among medical students is more urgent than ever. Results from our study suggest that aspects of medical education are unfavorable and disadvantageous for first-generation, URM and older medical students. Residency program directors continue to use USMLE test scores as a primary metric to screen applicants [ 60 ]. Therefore, poor performance on these exams has profound consequences on career trajectory which, in turn, may be impeding progress towards increasing diversity in medicine. The increased stress in first-generation students at the end of third year clerkships may be indicative of underlying psychological distress.
A deeper understanding of the interplay between sociodemographic characteristics and success in medical school, both psychosocially and academically, is paramount if we are to achieve diversity in medicine that matches that of the population and, ultimately, health equity. Our study looked at stress, which is just one possible cause for nontraditional students to be unsuccessful in medical school, and we looked at academic performance, which is just one possible measure of success or failure in medical school. The PSS-4 captures general stress at moments in time, but it tells us nothing about the quality of stress. Further investigation employing qualitative methods could elucidate sources of stress and factors undermining academic performance for nontraditional students. While USMLE exam performance continues to be a critical component of a student’s competitiveness for residency programs, it is closely followed by narrative evaluations [ 60 ]. Moreover, there is evidence that narrative evaluations, which are inherently subjective, are prone to both implicit and explicit bias, thereby adding another element of disadvantage for certain groups of nontraditional students [ 35 , 61 , 62 ]. To fully understand the experience of nontraditional medical students, exploring the effect of these evaluations, both internally at our institution and broadly across the US, is necessary. It is incumbent upon medical educators to support their students in meaningful ways and to promote the success of nontraditional students. This may be through individualized support, both academically and personally, flexible curricula to meet the needs of a diverse student body, or through system-level changes that lead to learning environments more favorable to nontraditional students.
Results from our study echo historic trends in academic performance for racial and ethnic minority students, with our URM students scoring significantly lower on the standardized preclinical NBME exams than their non-URM peers. Additionally, we present recent evidence of academic performance disparities based on age at matriculation, with our Age 30 + study group significantly underperforming on both institutional exams and national licensing exams. Our results also show significantly higher stress at the end of the third year of medical school for first-generation students, which may be evidence of imposter phenomenon, though further investigation is needed to make that conclusion definitively. Contrary to the literature, however, we did not find any differences based on socioeconomic disadvantage; we attribute this inconsistency to limitations imparted on our study by the nature of the study design itself.
Availability of data and materials
Data available upon request. Please send data requests to Edward Simanton PhD, [email protected].
Nontraditional student refers to students who are considered first-generation college students, underrepresented in medicine minority, socioeconomically disadvantaged, or took any path to medical school other than completing high school, then completing a bachelor’s degree 4 years later, then immediately matriculating to medical school.
Abbreviations
United States Medical Licensing Examination
- Underrepresented in medicine
National College Health Assessment
Perceived Stress Scale
Internal review board
First-generation college student
Socioeconomically Disadvantaged
Association of American Medical Colleges
United States
Continuing-generation college students
Socioeconomic status
Education-Occupation
Socioeconomic Status-Education Occupation
Matriculating Student Questionnaire
Perceived Stress Scale-4 (short form)
National Board of Medical Examiners
Clinical Knowledge
Statistical Package for Social Sciences
Continuing-generation college student
Perceived Stress Scale-4 (short form)
- Socioeconomic disadvantage
Step 2 Clinical Knowledge
Confidence interval
Medical College Admission Test
Grade-point average
Perceived Stress Scale-10 (10-item questionnaire)
Science, technology, engineering, and mathematics
Liaison Committee on Medical Education
Pisaniello MS, Asahina AT, Bacchi S, Wagner M, Perry SW, Wong M, Licinio J. Effect of medical student debt on mental health, academic performance and specialty choice: a systematic review. BMJ Open. 2019;9(7):e029980. https://doi.org/10.1136/bmjopen-2019-029980 .
Article Google Scholar
McKerrow I, Carney PA, Caretta-Weyer H, Furnari M, Miller Juve A. Trends in medical students’ stress, physical, and emotional health throughout training. Medical Education Online. 2020;25(1):1709278. https://doi.org/10.1080/10872981.2019.1709278 . https://www.tandfonline.com/doi/abs/10.1080/10872981.2019.1709278 .
Rajapuram N, Langness S, Marshall MR, Sammann A. Medical students in distress: The impact of gender, race, debt, and disability. PloS One. 2020;15(12):e0243250. https://doi.org/10.1371/journal.pone.0243250 . https://www.ncbi.nlm.nih.gov/pubmed/33270759 .
Dyrbye LN, West CP, Satele D, Boone S, Tan L, Sloan J, Shanafelt TD. Burnout Among U.S. Medical Students, Residents, and Early Career Physicians Relative to the General U.S. Population. Academic medicine. 2014;89(3):443–51. https://doi.org/10.1097/ACM.0000000000000134 . https://www.ncbi.nlm.nih.gov/pubmed/24448053 .
Dyrbye L, Shanafelt T. A narrative review on burnout experienced by medical students and residents. Med Educ. 2016;50(1):132–49. https://doi.org/10.1097/ACM.0000000000000134 . https://doi.org/10.1111/medu.12927 .
Harolds JA. Quality and Safety in Healthcare, Part LXVI: Contributing Causes of Poor Well-being in Medical Students. Clin Nucl Med. 2021;46(2):133–5. https://doi.org/10.1097/RLU.0000000000002969 . https://www.ncbi.nlm.nih.gov/pubmed/32108702 .
Committee on Systems Approaches to Improve Patient Care by Supporting Clinician Well-Being, National Academy of Medicine, National Academy of Sciences, Engineering, and Medicine: Taking action against clinician burnout : a systems approach to professional well-being: Washington, DC: The National Academies Press; 2019. https://doi.org/10.17226/25521 .
American College Health Association: ACHA-NCHA III: Graduate/Professional Student Reference Group Executive Summary Spring 2022. American College Health Association. 2022, https://www.acha.org/NCHA/ACHA-NCHA_Data/Publications_and_Reports/NCHA/Data/Reports_ACHA-NCHAIII.aspx .
Hill MR, Goicochea S, Merlo LJ. In their own words: stressors facing medical students in the millennial generation. Med Edu Online. 2018;23(1):1530558–10. https://doi.org/10.1080/10872981.2018.1530558 . https://www.tandfonline.com/doi/abs/10.1080/10872981.2018.1530558 .
Dyrbye LN, Thomas MR, Shanafelt TD. Systematic review of depression, anxiety, and other indicators of psychological distress among U.S. and Canadian medical students. Acad Med. 2006;81(4):354–73. https://doi.org/10.1097/00001888-200604000-00009 . https://www.ncbi.nlm.nih.gov/pubmed/16565188 .
Dyrbye LN, Sciolla AF, Dekhtyar M, Rajasekaran S, Allgood JA, Rea M, Knight AP, Haywood A, Smith S, Stephens MB. Medical school strategies to address student well-being: a national survey. Acad Med. 2019;94(6):861–8. https://doi.org/10.1097/ACM.0000000000002611 . https://www.ncbi.nlm.nih.gov/pubmed/30681453 .
Kligler B, Linde B, Katz NT. Becoming a doctor: a qualitative evaluation of challenges and opportunities in medical student wellness during the third year. Acad Med. 2013;88(4):535–40. https://doi.org/10.1097/ACM.0b013e3182860e6d . https://www.ncbi.nlm.nih.gov/pubmed/23425993 .
McMichael B, Lee A IV, Fallon B, Matusko N, Sandhu G. Racial and socioeconomic inequity in the financial stress of medical school. MedEdPublish. 2022;12:3. https://doi.org/10.12688/mep.17544.2 . https://search.proquest.com/docview/2718963370 .
Amirkhan JH, Manalo R, Velasco SE: Stress overload in first-generation college students: Implications for intervention. Psychol Serv. 2022:1–11. https://doi.org/10.1037/ser0000650 Advance online publication(-). https://www.ncbi.nlm.nih.gov/pubmed/35389674 .
Mason HRC, Winseman J, Marcellon R, Huamantl M, Ruiz C, Ayala EE. First-Generation medical student wellness in the United States. J Best Pract Health Prof Divers. 2018;11(2):96–106 ( https://www.jstor.org/stable/26894211 ).
Google Scholar
Stevens C, Liu CH, Chen JA. Racial/ethnic disparities in US college students’ experience: Discrimination as an impediment to academic performance. J Am Coll Health. 2018;66(7):665–73. https://doi.org/10.1080/07448481.2018.1452745 .
Smedley BD, Myers HF, Harrell SP. Minority-status stresses and the college adjustment of ethnic minority freshmen. J Higher Educ. 1993;64(4):434–52. https://doi.org/10.1080/00221546.1993.11778438 . https://www.tandfonline.com/doi/abs/10.1080/00221546.1993.11778438 .
Kick S, Adams L, O’Brien-Gonzales A. Unique Issues of Older Medical Students. Teach Learn Med. 2000;12(3):150–5. https://doi.org/10.1207/S15328015TLM1203_6 .
Furquim F, Glasener KM, Oster M, McCall BP, DesJardins SL. Navigating the Financial Aid Process: Borrowing Outcomes among First-Generation and Non-First-Generation Students. Ann Am Acad Polit SS. 2017;671(1):69–91. https://doi.org/10.1177/0002716217698119 . https://doi.org/10.1177/0002716217698119 .
Grayson MS, Newton DA, Thompson LF. Payback time: the associations of debt and income with medical student career choice. Med Educ. 2012;46(10):983–91. https://doi.org/10.1111/j.1365-2923.2012.04340.x . https://api.istex.fr/ark:/67375/WNG-MFLPNPP3-4/fulltext.pdf .
Thomas BR, Dockter N. Affirmative action and holistic review in medical school admissions: where we have been and where we are going. Acad Med. 2019;94(4):473–6. https://doi.org/10.1097/ACM.0000000000002482 . https://www.ncbi.nlm.nih.gov/pubmed/30277960 .
Walker KO, Moreno G, Grumbach K. The association among specialty, race, ethnicity, and practice location among California physicians in diverse specialties. J Natl Med Assoc. 2012;104(1):46–52. https://doi.org/10.1016/S0027-9684(15)30126-7 . https://www.clinicalkey.es/playcontent/1-s2.0-S0027968415301267 .
Garcia AN, Kuo T, Arangua L, Pérez-Stable EJ. Factors associated with medical school graduates’ intention to work with underserved populations: policy implications for advancing workforce diversity. Acad Med. 2018;93(1):82–9. https://doi.org/10.1097/ACM.0000000000001917 . https://www.ncbi.nlm.nih.gov/pubmed/28930761 .
Association of American Medical Colleges: A Healthier Future for All: The AAMC Strategic Plan. Association of American Medical Colleges; 2023. https://www.aamc.org/about-us/strategic-plan/healthier-future-all-aamc-strategic-plan .
Association of American Medical Colleges: Table A-26: First Generation Applicants, Acceptees, and Matriculants to U.S. MD-Granting Medical Schools, Academic Years 2020-2021 through 2022-2023. AAMC. AAMC 2022, https://www.aamc.org/data-reports/students-residents/data/2022-facts-applicants-and-matriculants-data .
Association of American Medical Colleges: Table A-14.3: Race/Ethnicity Responses (Alone and In Combination) of Matriculants to U.S. MD-Granting Medical Schools, 2019–2020 through 2023–2024. AAMC 2023, . https://www.aamc.org/media/8826/download .
Association of American Medical Colleges: Table A-24: Applicants, Acceptees, and Matriculants to U.S. MD-Granting Medical Schools by Socioeconomic Status (SES), Academic Years 2018–2019 through 2022–2023. AAMC 2022, . https://www.aamc.org/media/57171/download .
Association of American Medical Colleges: Matriculating Student Questionnaire: 2022 All Schools Summary Report. AAMC. 2022, https://www.aamc.org/data-reports/students-residents/report/matriculating-student-questionnaire-msq .
Youngclaus J, Roskovensky L. Analysis in Brief: an updated look at the economic diversity of US medical students. AAMC. 2018;18(5):1–3. https://www.aamc.org/data-reports/analysis-brief/report/updated-look-economic-diversity-us-medical-students .
Association of American Medical Colleges: Underrepresented in medicine definition. (n.d.)., https://www.aamc.org/what-we-do/equity-diversity-inclusion/underrepresented-in-medicine . Accessed 30 Oct 2022.
Warttig SL, Forshaw MJ, South J, White AK. New, normative, English-sample data for the Short Form Perceived Stress Scale (PSS-4). J Health Psychol. 2013;18(12):1617–28. https://doi.org/10.1177/1359105313508346 .
Cohen S, Kamarck T, Mermelstein R. A Global Measure of Perceived Stress. J Health Soc Behav. 1983;24(4):385–96. https://doi.org/10.2307/2136404 . https://www.jstor.org/stable/2136404 .
Association of American Medical Colleges: Medical School Year Two Questionnaire: 2022 All Schools Summary Report. AAMC. 2022, https://www.aamc.org/data-reports/students-residents/report/year-two-questionnaire-y2q .
Jones AC, Nichols AC, McNicholas CM, Stanford FC. Admissions is not enough: the racial achievement gap in medical education. Acad Med. 2021;96(2):176–81. https://doi.org/10.1097/ACM.0000000000003837 .
Teherani A, Hauer KE, Fernandez A, King TE, Lucey C. How small differences in assessed clinical performance amplify to large differences in grades and awards: a cascade with serious consequences for students underrepresented in medicine. Acad Med. 2018;93(9):1286–92. https://doi.org/10.1097/ACM.0000000000002323 . https://www.ncbi.nlm.nih.gov/pubmed/29923892 .
Poll-Hunter NI, Brown Z, Smith A, Starks SM, Gregory-Bass R, Robinson D, Cullins MD, Capers Q, Landry A, Bush A, Bellamy K, Lubin-Johnson N, Fluker CJ, Acosta DA, Young GH, Butts GC, Bright CM. Increasing the representation of black men in medicine by addressing systems factors. Acad Med. 2023;98(3):304–12. https://doi.org/10.1097/ACM.0000000000005070 . https://www.ncbi.nlm.nih.gov/pubmed/36538673 .
Rubright JD, Jodoin M, Barone MA. Examining demographics, prior academic performance, and United States medical licensing examination scores. Acad Med. 2019;94(3):364–70. https://doi.org/10.1097/ACM.0000000000002366 . https://www.ncbi.nlm.nih.gov/pubmed/30024473 .
Andriole DA, Jeffe DB. A National Cohort Study of U.S. Medical School Students Who Initially Failed Step 1 of the United States Medical Licensing Examination. Academic medicine. 2012;87(4):529–36. https://doi.org/10.1097/ACM.0b013e318248dd9c . https://www.ncbi.nlm.nih.gov/pubmed/22361789 .
Association of American Medical Colleges: Table A-18: MCAT Scores and GPAs for Applicants and Matriculants to U.S. MD-Granting Medical Schools by Race/Ethnicity, 2022–2023. AAMC. AAMC 2022, https://www.aamc.org/media/6066/download
Feil D, Kristian M, Mitchell N. Older medical students’ performances at McGill University. Acad Med. 1998;73(1):98–100. https://doi.org/10.1097/00001888-199801000-00020 .
Villwock JA, Sobin LB, Koester LA, Harris TM. Impostor syndrome and burnout among American medical students: a pilot study. Int J Med Educ. 2016;7:364–9. https://doi.org/10.5116/ijme.5801.eac4 . https://www.ncbi.nlm.nih.gov/pubmed/27802178 .
Levant B, Villwock JA, Manzardo AM. Impostorism in American medical students during early clinical training: gender differences and intercorrelating factors. Int J Med Educ. 2020;11:90–6. https://doi.org/10.5116/ijme.5e99.7aa2 .
Pratt IS, Harwood HB, Cavazos JT, Ditzfeld CP. Should I Stay or Should I Go? Retention in First-Generation College Students. J Coll Stud Retent Re Theory Pract. 2019;21(1):105–18. https://doi.org/10.1177/1521025117690868 . https://journals.sagepub.com/doi/full/10.1177/1521025117690868 .
Stebleton M, Soria K. Breaking down barriers: Academic obstacles of first-generation students at research universities. Learn Assist Rev. 2013;17(2):8–20. https://eric.ed.gov/?id=EJ1002281 .
Canning EA, LaCosse J, Kroeper KM, Murphy MC. Feeling like an imposter: the effect of perceived classroom competition on the daily psychological experiences of first-generation college students. Soc Psychol Pers Sci. 2020;11(5):647–57. https://doi.org/10.1177/1948550619882032 .
Crum AJ, Salovey P, Achor S. Rethinking Stress: The Role of Mindsets in Determining the Stress Response. J Pers Soc Psychol. 2013;104(4):716–33. https://doi.org/10.1037/a0031201 . http://psycnet.apa.org/journals/psp/104/4/716 .
Goldie J. The formation of professional identity in medical students: Considerations for educators. Med Teach. 2012;34(9):e641–8. https://doi.org/10.3109/0142159X.2012.687476 .
Matthews R, Smith-Han K, Nicholson H. From physiotherapy to the army: negotiating previously developed professional identities in mature medical students. Adv in Health Sci Educ. 2020;25(3):607–27. https://doi.org/10.1007/s10459-019-09942-0 .
Cohen JJ, Gabriel BA, Terrell C. The case for diversity in the health care workforce. Health Affairs. 2002;21(5):90–102. https://doi.org/10.1377/hlthaff.21.5.90 . http://content.healthaffairs.org/cgi/content/abstract/21/5/90 .
Boatright DH, Samuels EA, Cramer L, Cross J, Desai M, Latimore D, Gross CP. Association between the liaison committee on medical education’s diversity standards and changes in percentage of medical student sex, race, and ethnicity. JAMA. 2018;320(21):2267–9. https://doi.org/10.1001/jama.2018.13705 .
Association of American Medical Colleges: Liaison Committee on Medical Education (LCME) Standards on Diversity. 2009, https://health.usf.edu/~/media/Files/Medicine/MD%20Program/Diversity/LCMEStandardsonDiversity1.ashx?la=en .
Liaison Committee on Medical Education: Functions and Structure of a Medical School. AAMC 2023, . https://lcme.org/publications/ .
Grbic D, Morrison E, Sondheimer HM, Conrad SS, Milem JF. The Association between a holistic review in admissions workshop and the diversity of accepted applicants and students matriculating to medical school. Academic medicine. 2019;94(3):396–403. https://doi.org/10.1097/ACM.0000000000002446 . https://www.ncbi.nlm.nih.gov/pubmed/30188373 .
Bright CM, Price MA, Morgan RC, Bailey RK. The Report of the W. Montague Cobb /NMA Health Institute Consensus Panel on the Plight of Underrepresented Minorities in Medical Education. J Natl Med Assoc. 2018;110(6):614–23. https://doi.org/10.1016/j.jnma.2018.03.012 . https://www.proquest.com/scholarly-journals/report-w-montague-cobb-nma-health-institute/docview/2188190276/se-2?accountid=3611 .
Dandar V, Fair M, Steinecke A, Sweeny N, Mallory T: The Power of Collective Action: Assessing and Advancing Diversity, Equity, and Inclusion Efforts at AAMC Medical Schools. AAMC 2022, https://www.aamc.org/data-reports/report/assessing-and-advancing-dei-efforts-aamc-medical-schools .
Talamantes E, Jerant A, Henderson MC, Griffin E, Fancher T, Grbic D, Moreno G, Franks P. Community college pathways to medical school and family medicine residency training. Ann Family Med. 2018;16(4):302–7. https://doi.org/10.1370/afm.2270 . https://www.clinicalkey.es/playcontent/1-s2.0-S1544170919300071 .
Ma J, Baum S: Research on the Student-centered Talent Cultivation in Universities and Colleges. Atlantis Press; 2016. https://doi.org/10.2991/icessms-16.2017.111 .
Agency for Healthcare Research and Quality: 2022 National Healthcare Quality and Disparities Report. U.S. Department of Health and Human Services. U.S. Department of Health and Human Services 2022, 22(23)-0030 https://www.ahrq.gov/sites/default/files/wysiwyg/research/findings/nhqrdr/2022qdr-final-es.pdf .
Reynolds R, Ritashree Chakrabarti, Chylak D, Jones K, Iacobucci W, Dall T: The Complexities of Physician Supply and Demand: Projections From 2019 to 2034. IHS Markit Ltd. & AAMC. IHS Markit Ltd. 2021, https://www.search.datacite.org/works/10.13140/rg.2.2.29404.92808 .
National Resident Matching Program Data Release and Research Committee: Results of the 2021 NRMP Program Director Survey. National Resident Matching Program. National Resident Matching Program 2021, https://www.nrmp.org/wp-content/uploads/2021/11/2021-PD-Survey-Report-for-WWW.pdf .
Rojek AE, Khanna R, Yim JWL, Gardner R, Lisker S, Hauer KE, Lucey C, Sarkar U. Differences in Narrative Language in Evaluations of Medical Students by Gender and Under-represented Minority Status. J Gen Intern Med. 2019;34(5):684–91. https://doi.org/10.1007/s11606-019-04889-9 . https://link.springer.com/article/10.1007/s11606-019-04889-9 .
Low D, Pollack SW, Liao ZC, Maestas R, Kirven LE, Eacker AM, Morales LS. Racial/Ethnic disparities in clinical grading in medical school. Teach Learn Med. 2019;31(5):487–96. https://doi.org/10.1080/10401334.2019.1597724 . https://www.tandfonline.com/doi/abs/10.1080/10401334.2019.1597724 .
Download references
Acknowledgements
The authors would like to thank Dr. Dale Netski, Director of Medical Student Research, for his review of the manuscript, and Kathryn M. Houk, Undergraduate Medical Education Librarian, for her assistance with resource utilization and citation management software.
No funding was accepted to assist with this study or preparation of this manuscript.
Author information
Authors and affiliations.
Kirk Kerkorian School of Medicine at UNLV, 625 Shadow Lane, Las Vegas, NV, 89106, USA
Danielle Eames, Shelby Thomas, Kaden Norman, Edward Simanton & Anne Weisman
You can also search for this author in PubMed Google Scholar
Contributions
DE made substantial contributions to literature review, methods conception, data analysis, preparation and revision of all parts of the manuscript. ST made substantial contributions to literature review, data analysis, discussion, conclusion, review and editing of all parts of the manuscript. KN made substantial contributions to literature review, data analysis, and editing of all parts of the manuscript. ES made substantial contributions to methods conception, data retrieval from institutional databases, data analysis, review and editing of all parts of the manuscript. AW made substantial contributions to methods conception, data analysis, review and editing of all parts of the manuscript. All authors reviewed and approved the final version of this manuscript.
Authors’ information
DE is a medical student at the Kirk Kerkorian School of Medicine at UNLV, graduating May 2024.
ST is a medical student at the Kirk Kerkorian School of Medicine at UNLV, graduating May 2024.
KN is a medical student at the Kirk Kerkorian School of Medicine at UNLV, graduating May 2026.
ES is Director of Educational Outcomes and Assessment at Kirk Kerkorian School of Medicine at UNLV.
AW is Director of Well-Being & Integrative Medicine and Associate Professor of Medical Education at Kirk Kerkorian School of Medicine at UNLV.
Corresponding author
Correspondence to Danielle Eames .
Ethics declarations
Ethics approval and consent to participate.
This study was approved by the Biomedical Institutional Review Board of the University of Nevada Las Vegas. All experimental protocols, including waiver of informed consent, were approved by the Biomedical Institutional Review Board of the University of Nevada Las Vegas ethics committee. All methods were carried out in accordance with relevant guidelines and regulations and in accordance with the IRB-approved protocol “School of Medicine use of program evaluation data for research” [protocol number 1030906–1], dated April 3rd, 2017.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary material 1., supplementary material 2., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Eames, D., Thomas, S., Norman, K. et al. Sociodemographic disadvantage in the burden of stress and academic performance in medical school: implications for diversity in medicine. BMC Med Educ 24 , 348 (2024). https://doi.org/10.1186/s12909-024-05263-y
Download citation
Received : 02 July 2023
Accepted : 05 March 2024
Published : 29 March 2024
DOI : https://doi.org/10.1186/s12909-024-05263-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Nontraditional
- Medical student
- Sociodemographic
- First-generation
- Older students
- Diversity in medicine
BMC Medical Education
ISSN: 1472-6920
- Submission enquiries: [email protected]
- General enquiries: [email protected]
- Search Menu
- Advance Articles
- Collections
- Focus Collections
- Teaching Tools in Plant Biology
- Browse by cover
- High-Impact Research
- Author Guidelines
- Submission Site
- Open Access Options
- Self-Archiving Policy
- Why Publish with Us?
- About The Plant Cell
- About The American Society of Plant Biologists
- Editorial Board
- Advertising & Corporate Services
- Journals on Oxford Academic
- Books on Oxford Academic

Article Contents
The s-acylation cycle of transcription factor mtnac80 influences cold stress responses in medicago truncatula.
Qinyi Ye and Lihua Zheng Equal contribution
- Article contents
- Figures & tables
- Supplementary Data
Qinyi Ye, Lihua Zheng, Peng Liu, Qianwen Liu, Tuo Ji, Jinling Liu, Yajuan Gao, Li Liu, Jiangli Dong, Tao Wang, The S-acylation cycle of transcription factor MtNAC80 influences cold stress responses in Medicago truncatula , The Plant Cell , 2024;, koae103, https://doi.org/10.1093/plcell/koae103
- Permissions Icon Permissions
S-acylation is a reversible post-translational modification catalyzed by protein S-acyltransferases (PATs), and acyl protein thioesterases (APTs) mediate de-S-acylation. Although many proteins are S-acylated, how the S-acylation cycle modulates specific biological functions in plants is poorly understood. In this study, we report that the S-acylation cycle of transcription factor MtNAC80 is involved in the Medicago truncatula cold stress response. Under normal conditions, MtNAC80 localized to membranes through MtPAT9-induced S-acylation. In contrast, under cold stress conditions, MtNAC80 translocated to the nucleus through de-S-acylation mediated by thioesterases such as MtAPT1. MtNAC80 functions in the nucleus by directly binding the promoter of the glutathione S -transferase gene MtGSTU1 and promoting its expression, which enables plants to survive under cold stress by removing excess malondialdehyde and H 2 O 2 . Our findings reveal an important function of the S-acylation cycle in plants and provide insight into stress response and tolerance mechanisms.
Author notes
Supplementary data, email alerts, citing articles via.
- Recommend to Your Librarian
- Advertising & Corporate Services
- Awards & Funding
- Plant Science Today
- Plant Biology Meeting
- Meeting Management Services
- Plant Science Research Weekly
- Taproot: A Plantae Podcast
Affiliations
- Online ISSN 1532-298X
- Print ISSN 1040-4651
- Copyright © 2024 American Society of Plant Biologists
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.

StatPearls [Internet].
Stress management.
Mary Worthen ; Elizabeth Cash .
Affiliations
Last Update: August 14, 2023 .
- Introduction
Effective techniques for stress management are varied. They typically include behaviors that improve physical health, such as nutrition and exercise, but may also incorporate strategies that improve cognitive and emotional functioning. The stress-reduction approach based on mindfulness practices has recently enjoyed an explosion of interest from a variety of healthcare and epidemiological researchers. [1] [2] [3] The concept of mindfulness, which originates from practices of Buddhism, is defined as a focused awareness of one’s experience, and purposeful and nonjudgmental focus on the present moment. [4] Structured interventions, such as the Mindfulness-Based Stress Reduction (MBSR) program, provide participants with the opportunity to learn breathing meditation, body scanning techniques, and gentle, yoga-inspired physical exercises. [5] With practice, individuals learn to process emotions, thoughts, and sensations as they arise. Individuals learn to modify their reflexive conditioning from automatically reacting or worrying about the future to a more adaptive, measured response with greater awareness of the present moment. [6] The literature is replete with evidence suggesting that, with practice, individuals can become more mindful, increasing their capacity to fully process emotions, thoughts, and sensations as they arise. [7] [8] [9] [10] [11] [12] MBSR interventions have been adapted to a wide variety of individuals, from those suffering from chronic or debilitating health conditions to healthy undergraduate or medical students. Randomized controlled trials of MBSR interventions have demonstrated improvements to psychological and physiological processes with relevance to health outcomes and improved stress management.
Some individuals have a greater innate, or trait, capacity for mindfulness. These individuals, who have not participated in mindfulness-training interventions, tend to experience better physical health, report fewer physiological symptoms such as pain, and utilize fewer healthcare resources. [13] Trait mindfulness has been associated with lower ratings of anxiety and depression in a variety of medical and non-medical populations. [14] Trait mindfulness may emerge from a genetic predisposition. A recent epidemiological study of adolescent twins revealed that trait mindfulness was 32% heritable. [15] The same study also revealed that 66% of the variance in trait mindfulness was due to environmental factors, suggesting that is also a skill that can be learned. In fact, an MBSR study in university undergraduates revealed that, while increases in mindfulness and psychological outcomes can be observed in participants as a whole, effects may be more pronounced among individuals higher in trait mindfulness at study entry. [16] These data substantiate the utility of mindfulness training, even for high-trait individuals.
- Issues of Concern
Standard MBSR Programs
Standard MBSR programs have demonstrated potential to ameliorate physiological dysregulation, including attenuated hypothalamic-pituitary-adrenal (HPA) axis activation, autonomic activation, and inflammation. [17] [18] A study of healthy adult males who endorsed a higher capacity for present-moment focus found they also exhibited reduced emotional distress and autonomic (heart rate) reactivity when exposed to hypoxic conditions. [19] Neural correlates may underlie relationships between mindfulness practices and central nervous system (CNS) function reported in the literature. Higher trait mindfulness positively correlates with activity in the anterior cingulate and prefrontal cortices in healthy adults, both of which demonstrate reduced activity in studies of individuals suffering from anxiety and depressive disorders. [20] Levels of trait mindfulness also correlate with grey matter volume reductions in the amygdala and caudate in healthy adults and greater volume in bilateral gyri of adults with generalized anxiety disorder. [20] Likewise, studies also demonstrate that mindfulness training results in increased blood flow in the amygdala and hippocampal regions among breast cancer patients and increased grey matter concentrations in the norepinephrine and serotonin systems in the brain of in healthy adults. [21] [22] This evidence for shared neural circuitry suggests at least partial mechanisms by which mindful approaches may be beneficial for individuals who are experiencing prolonged psychological distress or difficulty managing stress.
Mindfulness-Based Stress Reduction
Mindfulness-Based Stress Reduction (MBSR) was initially designed to relieve suffering among chronic pain patients by teaching skills that cultivate present moment-focused, non-judgmental awareness. [5] Three formal mindfulness techniques are taught over eight weeks: The “body scan,” an attention-focusing technique; gentle yoga; and sitting meditation. The body scan is performed supine with attention directed systematically and non-judgmentally through regions of the body, encouraging relaxed awareness and acceptance of proprioceptive and interoceptive sensation. Hatha yoga consists of gentle movement and stretching sequences that promote awareness of movement and position. Sitting meditation helps develop a stable cognitive perspective from which to observe mental events with openness and acceptance without becoming caught up in distressing thoughts or feelings. Informal mindfulness practice is also encouraged: Participants cultivate an awareness of the present moment, whether perceived as pleasant or unpleasant. The intent is to help participants become engaged with the experiences of each moment as they arise and learn to face stressful events in a way that is skillfully responsive rather than habitually reactive. With practice, individuals learn to modify their reflexive conditioning from automatically reacting or worrying about the future to a more adaptive, measured response with greater awareness of the present moment.
Traditional MBSR Interventions
Traditional MBSR interventions can easily be performed without the use of specialized equipment. Many group administrators and practitioners prefer to have materials handy to help modify movement-based exercises and yoga poses for those with physical limitations. Typically a yoga mat, blocks, and strap are sufficient. Studies of MBSR group interventions have suggested that limiting group size to less than 20 individuals is preferable to build a cohesive dynamic between practitioners (see Lehrer, Woolfolk, and Sime, Principles and practice of stress management. New York: Guilford). [23] Guided practices, including the body scan and sitting meditations, are available on electronic media or for download from several sources. There is also an abundance of freely available apps and podcasts that offer mindfulness teachings, guided and unguided timed sitting and supine meditations, and guided mindfulness practices to listen to during activities such as exercising or cleaning. Most are available for free or for a range of modest costs. Some offer free trials, while some apps and many quality podcasts are totally free. Encourage interested individuals to try out a few different options and find what works best for them. Practitioners also may find that different approaches are more preferable, depending on the day, stress level, and practice time available. We caution individuals to avoid listening to guided sessions or podcasts while driving, as increased drowsiness may occur.
Mindfulness-based interventions may be optimized to enhance pre-existing practitioner strengths and address specific stress-management needs. Among cancer patients, a benefit was observed after an MBSR program incorporated a shortened length of meditation assignments, reduced frequency of group meetings, and adjustments of the physical movements taught (e.g., yoga poses). Similar modifications have been considered for other cancer patient samples, including changing the usual mindful (raisin) eating exercise to utilize a liquid for those who have trouble eating or swallowing, omitting the day-long silent retreat if thought to be too taxing or inappropriate for the population, adjusting physical movements to account for any injury or pain that may be present, and employing an individual (versus group) format to better incorporate session scheduling with medical appointments. [24] Given the similar improvements to psychological measures when compared to standard MBSR program outcomes, modified interventions may not be an inferior approach to increasing mindfulness practices among participants. These strategies may be advantageous with regard to implementation and utilization by practitioners who are experiencing busy daily activities and have limited flexibility to facilitate adding a new routine to their daily lives.
- Clinical Significance
Several authors have offered thoughtful conceptualizations of mechanisms by which mindfulness may reduce stress and ameliorate illness symptoms. [25] Theoretical models focus on metacognitive factors, explore mindfulness in the context of a widely accepted model of stress and coping, and contrast this practice, which stems from Eastern tradition and includes Western psychology. [26] [27] Other recent work has highlighted the need to examine process variables related to the techniques used, individual meditation practice, social factors, and other aspects of MBSR. [28]
Novice mindfulness practitioners also engage in "informal" practice as they learn to observe their own thoughts and sensations and explore a new stance as a nonjudgmental observer of their own life. Attending to one’s own experience may set up a dynamic cognitive interaction that can facilitate a capacity to respond to ongoing experiences as if they are occurring for the first time, typically referred to as "beginner’s mind." This interrupts the automatic processes of relying on previously conditioned stress reactions. Paradoxically, positive changes seem especially likely to occur when one can let go of the struggle of trying to change or control the process. This perspective lies at the core of empirically validated acceptance-based intervention model. A focus on the present moment can potentially help decondition habitual reaction patterns and increase response flexibility. From a cognitive perspective, this suggests that viewing present circumstances as new and unique experiences increases one’s capacity for generating multiple alternative response options. Mindfulness also may address cognitive-behavioral factors, such as self-focused attention, experiential avoidance, and perceived control. [29] [30]
- Review Questions
- Access free multiple choice questions on this topic.
- Comment on this article.
Disclosure: Mary Worthen declares no relevant financial relationships with ineligible companies.
Disclosure: Elizabeth Cash declares no relevant financial relationships with ineligible companies.
This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) ( http://creativecommons.org/licenses/by-nc-nd/4.0/ ), which permits others to distribute the work, provided that the article is not altered or used commercially. You are not required to obtain permission to distribute this article, provided that you credit the author and journal.
- Cite this Page Worthen M, Cash E. Stress Management. [Updated 2023 Aug 14]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
In this Page
Bulk download.
- Bulk download StatPearls data from FTP
Related information
- PMC PubMed Central citations
- PubMed Links to PubMed
Similar articles in PubMed
- The effectiveness of mindfulness based programs in reducing stress experienced by nurses in adult hospital settings: a systematic review of quantitative evidence protocol. [JBI Database System Rev Implem...] The effectiveness of mindfulness based programs in reducing stress experienced by nurses in adult hospital settings: a systematic review of quantitative evidence protocol. Botha E, Gwin T, Purpora C. JBI Database System Rev Implement Rep. 2015 Oct; 13(10):21-9.
- Lifestyle Mindfulness In Clinical Practice. [StatPearls. 2024] Lifestyle Mindfulness In Clinical Practice. Srour RA, Keyes D. StatPearls. 2024 Jan
- Review Mindfulness and bodily distress. [Dan Med J. 2012] Review Mindfulness and bodily distress. Fjorback LO. Dan Med J. 2012 Nov; 59(11):B4547.
- A pilot study comparing the effects of mindfulness-based and cognitive-behavioral stress reduction. [J Altern Complement Med. 2008] A pilot study comparing the effects of mindfulness-based and cognitive-behavioral stress reduction. Smith BW, Shelley BM, Dalen J, Wiggins K, Tooley E, Bernard J. J Altern Complement Med. 2008 Apr; 14(3):251-8.
- Review [The history of Mindfulness put to the test of current scientific data: unresolved questions]. [Encephale. 2014] Review [The history of Mindfulness put to the test of current scientific data: unresolved questions]. Trousselard M, Steiler D, Claverie D, Canini F. Encephale. 2014 Dec; 40(6):474-80. Epub 2014 Sep 5.
Recent Activity
- Stress Management - StatPearls Stress Management - StatPearls
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers

IMAGES
VIDEO
COMMENTS
Stress and Memory. Memory is one of the important functional aspects of the CNS and it is categorized as sensory, short term, and long-term. Short term memory is dependent on the function of the frontal and parietal lobes, while long-term memory depends on the function of large areas of the brain (Wood et al., 2000[]).However, total function of memory and the conversion of short term memory to ...
Stress is an inescapable element of the modern age. Instances of untreated stress may lead to a reduction in the individual's health, well-being and socio-economic situation. ... Melillo P., Pecchia L., Santamaria L., James C. Detection of mental stress due to oral academic examination via ultra-short-term HRV analysis; Proceedings of the ...
These hormones intensify your concentration, ability to react, and strength. Also, your heart rate and blood pressure increase, and your immune system and memory are shaper. After you have dealt with the short-term stress, your body returns to normal. Chronic or long-term stress, however, poses a problem. If you repeatedly face challenges and ...
The human stress response is an additional homeostatic mechanism that provides a better chance of survival when the body is under threat and mobilizes neural and hormonal networks to optimize ...
Abstract. The cumulative science linking stress to negative health outcomes is vast. Stress can affect health directly, through autonomic and neuroendocrine responses, but also indirectly, through changes in health behaviors. In this review, we present a brief overview of ( a) why we should be interested in stress in the context of health; ( b ...
About the Journal. Stress & Health is an international forum for disseminating cutting-edge theoretical and empirical research that significantly advances understanding of the relationship between stress and health and well-being in humans. Fast online publication with your accepted manuscript publishing online within a week, with manuscript ...
Effective techniques for stress management are varied. They typically include behaviors that improve physical health, such as nutrition and exercise, but may also incorporate strategies that improve cognitive and emotional functioning. The stress-reduction approach based on mindfulness practices has recently enjoyed an explosion of interest ...
The editorial focus of the International Journal of Stress Management® ( IJSM) is the assessment, management, and treatment of stress and trauma, whether emotional, cognitive, behavioral, or physiological. Personal, occupational, organizational, and societal issues relevant to stress identification and management are also covered.
Chronic Stress is an open access, peer reviewed international journal publishing original and review articles related to all aspects of stress, including preclinical and clinical studies of stress-related psychiatric disorders (e.g. mood, anxiety, … | View full journal description. This journal is a member of the Committee on Publication ...
Background In the case of people who carry an increased number of anxiety traits and maladaptive coping strategies, psychosocial stressors may further increase the level of perceived stress they experience. In our research study, we aimed to examine the levels of perceived stress and health anxiety as well as coping styles among university students amid the COVID-19 pandemic. Methods A cross ...
Positive effects of acute stress on immune responses were reported in early research into psychoneuroimmunological interactions (PNIs) (Blecha, Barry, & Kelley, 1982) and the literature has been regularly reviewed (Dantzer & Kelley, 1989; Dhabhar, 2009, 2014; Glaser & Kiecolt-Glaser, 2005 ). Dhabhar ( 2018) has proposed the concept of the ...
Mindfulness-based stress reduction 36 is a widely disseminated and frequently cited example of mindfulness training that has been shown to reduce stress, depression, and anxiety. 49,50 Mindfulness-based stress reduction programs have been widely researched and positive results reported among an array of clinical and nonclinical populations, including cancer patients, 51-53 mixed illness ...
Burnout and stress are at all-time highs across professions, and among already strained health care workers, they are exacerbated by the politicization of mask-wearing and other unrelenting stressors. ... A June 2021 article in The Washington Post highlighted that companies of varying sizes and in many industries are finding new ways to ensure ...
This article describes best practices in stress measurement, detailing which dimensions of stressor exposures and stress responses to capture, and how. We describe when to use psychological versus physiological indicators of stress. ... We encourage the adoption of more precise language when writing about stress in academic papers, more careful ...
6. Conclusions and implications. This study found that Self-inflicted Stress, time management stress, group work stress, and performance stress were predictors of mental health, supporting the hypothesis that there is a negative relationship between academic stress and the mental health of university students.
Abstract. Life stress is a central construct in many models of human health and disease. The present article reviews research on stress and health, with a focus on (a) how life stress has been conceptualized and measured over time, (b) recent evidence linking stress and disease, and (c) mechanisms that might underlie these effects.
Stress. Stress is a normal reaction to everyday pressures, but can become unhealthy when it upsets your day-to-day functioning. Stress involves changes affecting nearly every system of the body, influencing how people feel and behave. By causing mind-body changes, stress contributes directly to psychological and physiological disorder and ...
Methods. A single author (MP) searched PubMed and Google Scholar for peer-reviewed articles published at any time in English. Search terms included academic, school, university, stress, mental health, depression, anxiety, youth, young people, resilience, stress management, stress education, substance use, sleep, drop-out, physical health with a combination of any and/or all of the preceding terms.
Research has shown that being a medical student is associated with a greater degree of perceived stress, reduced overall wellbeing [1,2,3], higher rates of depression, anxiety, and burnout [4,5,6,7] and that these experiences can negatively impact academic performance [1,2,3, 8].The 2022 National College Health Assessment (NCHA) survey, which collects data on factors that students perceive to ...
Academic stress may be the single most dominant stress factor that affects the mental well-being of college students. Some groups of students may experience more stress than others, and the coronavirus disease 19 (COVID-19) pandemic could further complicate the stress response. We surveyed 843 college students and evaluated whether academic ...
Studies on JD-R in HEIs have earlier primarily focused only on academic work stress. The changing landscape of the roles and expectations from faculty members in HEIs demands a revisit of the theory. The present study recognises non-academic work as a demand requiring additional resources and thoughtful planning. The study identifies stressors ...
In contrast, under cold stress conditions, MtNAC80 translocated to the nucleus through de-S-acylation mediated by thioesterases such as MtAPT1. MtNAC80 functions in the nucleus by directly binding the promoter of the glutathione S -transferase gene MtGSTU1 and promoting its expression, which enables plants to survive under cold stress by ...
Effective techniques for stress management are varied. They typically include behaviors that improve physical health, such as nutrition and exercise, but may also incorporate strategies that improve cognitive and emotional functioning. The stress-reduction approach based on mindfulness practices has recently enjoyed an explosion of interest from a variety of healthcare and epidemiological ...