

Kohlrausch Law
Kohlrausch Law relates to the limiting molar conductivity of an electrolyte to its constituent ions.
It basically states that the limiting molar conductivity of an electrolyte is equal to the sum of individual limiting molar conductivities of the cations and anions which make up the electrolyte.
Limiting Molar Conductivity? The molar conductivity of a solution at infinite dilution is known as limiting molar conductivity
This law is also popularly known as the Kohlrausch Law of Independent Migration.
Kohlrausch law and its applications are very important in the study of dilute solutions and also in the study of electrochemical cells . This law is mainly used to find the limiting conductivity of a weak electrolyte, among other important applications.
An example of this law is the limiting conductivity of sulphuric acid (H 2 SO 4 ). The limiting molar conductivity of sulphuric acid is equal to the sum of the limiting conductivities of hydrogen cation and sulphate anion.
The mathematical representation of the above statement is,
Here, \(\lambda^{\infty}\) is the limiting molar conductivity .
Friedrich Kohlrausch , a german physicist, gave this law in 1875-1879 and was thus named after him.
Kohlrausch played an important part in the development of physical chemistry and was an influential researcher of electrochemistry during his time.
The experiments which he used in order to develop the law of independent migration were used by famous and important chemists like Arrhenius , Ostwald and Van’t Hoff , who devised the Ionist theory and were called the founders of physical chemistry.

Kohlrausch Law Explained
The law of independent migration of ions states that the limiting molar conductivity of an electrolyte (i.e, the conductivity of an electrolyte at infinite dilution when all of it is dissociated into ions), is equal to the sum of the amount of its each constituent ion’s limiting molar conductivity .
The general case of this law is any random electrolyte A x B y .
The mathematical representation of the limiting molar conductivity of A x B y is,
\(\lambda^{\infty}_{A_xB_y} = 2\lambda^{\infty}_{A^{+y}} + \lambda^{\infty}_{B^{-x}}\)
When a pair of electrolytes have the same cation then the difference in their limiting molar conductivities does not depend on the cation and is only affected by a change in the anion.
The above statement is also true for electrolytes with the same anion.
Let us consider two pairs of electrolytes with common cations A and D in each pair, then the difference between their limiting molar conductivities is not affected by A or D. This can be mathematically represented as,
\(\lambda^{\infty}_{AB} – \lambda^{\infty}_{AC} = \lambda^{\infty}_{DB} – \lambda^{\infty}_{DC}\)
Here, \(\lambda^{\infty}_{AB}\) is limiting molar conductivity of AB \(\lambda^{\infty}_{AC}\) is limiting molar conductivity of AC \(\lambda^{\infty}_{DB}\) is limiting molar conductivity of DB \(\lambda^{\infty}_{DC}\) is limiting molar conductivity of DC
Applications of Kohlrausch Law
- It is used to calculate the dissociation constant of an electrolyte.
- It is used to calculate the limiting molar conductivity of a weak electrolyte.
- The degrees of dissociation of weak electrolytes are also found using this law.
- Solubility constants of various salts are also calculated using this law.
- It is also used in the calculation of the cell potential in various electrochemical cells.
Example Problems
Question 1. Calculate the limiting molar conductivity of sodium sulphate(Na 2 SO 4 ). If the limiting molar conductivity of \(Na^{+}\) is \(50.1 S.cm^2/mol\) and \(\frac{1}{2}SO_{4}^{2-}\) is \(80.0 S.cm^2/mol\).
Solution. Given, \(\lambda^{\infty}_{Na^{+}} = 50.1 S.cm^2/mol\) \(\frac{1}{2}\lambda^{\infty}_{SO_{4}^{2-}} = 80.0 S.cm^2/mol\)
According to Kohlrausch law of independent migration of ions,
\(\lambda^{\infty}_{Na_2SO_4} = 2\lambda^{\infty}_{Na^{+}} + 2*(\frac{1}{2}\lambda^{\infty}_{SO{4}^{2-}})\)
\(\lambda^{\infty}_{Na_2SO_4} = 2(50.1) + 2*80\)
=> \(\lambda^{\infty}_{Na_2SO_4} = 260.2 S.cm^2/mol\)
So, the limiting molar conductivity of sodium sulphate is \(260.2 S.cm^2/mol\).
Question 2. Calculate the limiting molar conductivity of CH 3 COOH which is a weak electrolyte. The molar conductivities of CH 3 COONa, HCl and NaCl at infinite dilution are \(90.1 S.cm^2/mol\), \(426.16 S.cm^2/mol\) and \(126.45 S.cm^2/mol\) respectively.
Solution. Given, \(\lambda^{\infty}_{CH_3COONa} = 90.1 S.cm^2/mol\) \(\lambda^{\infty}_{HCL} = 426.16 S.cm^2/mol\) \(\lambda^{\infty}_{NaCl} = 126.45 S.cm^2/mol\)
\(\lambda^{\infty}_{CH_3COOH} = \lambda^{\infty}_{CH_3COONa} + \lambda^{\infty}_{HCL} – \lambda^{\infty}_{NaCl}\)
\(\lambda^{\infty}_{CH_3COOH} = 91 + 426.16 – 126.45\)
=> \(\lambda^{\infty}_{CH_3COOH} = 390.71 S.cm^2/mol\)
So, the limiting molar conductivity of Acetic acid(CH 3 COOH) is, \(390.71 S.cm^2/mol\).
Friedrich Kohlrausch discovered this law from observing experimental data of conductivities of various electrolytes.
This law is used to calculate the limiting molar conductivity, degree of dissociation and dissociation constant of weak electrolytes. It is also used in the measurement of the solubility of a salt.
It is the law that gives the relationship between the limiting molar conductivity of an electrolyte and its constituent ions. It states that the limiting molar conductivity of an electrolyte is equal to sum of the amounts of limiting molar conductivities of the ions which it splits into after dilution. The mathematical relation is, \(\lambda^{\infty}_{A_xB_y} = 2\lambda^{\infty}_{A^{+y}} + \lambda^{\infty}_{B^{-x}}\)
This law was discovered by Kohlrausch in his experiments with dilute solutions. He backed up his law by the observation that, when a pair of different electrolytes have the same anode(or cathode) then the difference in their limiting molar conductivities is independent of the nature/type of the common anode(or cathode).
About The Author

Leave a Comment Cancel Reply
You must be logged in to post a comment.

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

8.10.9D: Ionic migration
- Last updated
- Save as PDF
- Page ID 20112

- Stephen Lower
- Simon Fraser University
The motion of ions in solution is mainly random
The conductance of an electrolytic solution results from the movement of the ions it contains as they migrate toward the appropriate electrodes. But the picture we tend to have in our minds of these ions moving in a orderly, direct march toward an electrode is wildly mistaken. The thermally-induced random motions of molecules is known as diffusion . The term migration refers specifically to the movement of ions due to an externally-applied electrostatic field.
The average thermal energy at temperatures within water's liquid range (given by RT ) is sufficiently large to dominate the movement of ions even in the presence of an applied electric field. This means that the ions, together with the water molecules surrounding them, are engaged in a wild dance as they are buffeted about by thermal motions (which include Brownian motion).
If we now apply an external electric field to the solution, the chaotic motion of each ion is supplemented by an occasional jump in the direction dictated by the interaction between the ionic charge and the field. But this is really a surprisingly tiny effect:
It can be shown that in a typical electric field of 1 volt/cm, a given ion will experience only about one field-directed (non-random) jump for every 10 5 random jumps. This translates into an average migration velocity of roughly 10 –7 m sec –1 (10 –4 mm sec –1 ). Given that the radius of the H 2 O molecule is close to 10 –10 m, it follows that about 1000 such jumps are required to advance beyond a single solvent molecule!
The ions migrate Independently
All ionic solutions contain at least two kinds of ions (a cation and an anion), but may contain others as well. In the late 1870's, the physicist Friedrich Kohlrausch noticed that the limiting equivalent conductivities of salts that share a common ion exhibit constant differences.
These differences represent the differences in the conductivities of the ions that are not shared between the two salts. The fact that these differences are identical for two pairs of salts such as KCl/LiCl and KNO 3 /LiNO 3 tells us that the mobilities of the non-common ions K + and LI + are not affected by the accompanying anions.
Kohlrausch's law greatly simplifies estimates of Λ 0
This principle is known as Kohlrausch's law of independent migration , which states that in the limit of infinite dilution ,
Each ionic species makes a contribution to the conductivity of the solution that depends only on the nature of that particular ion, and is independent of the other ions present.
Kohlrausch's law can be expressed as
Λ 0 = Σ λ 0 + + Σ λ 0 –
This means that we can assign a limiting equivalent conductivity λ 0 to each kind of ion:
Just as a compact table of thermodynamic data enables us to predict the chemical properties of a very large number of compounds, this compilation of equivalent conductivities of twenty different species yields reliable estimates of the of Λ 0 values for five times that number of salts.
We can now estimate weak electrolyte limiting conductivities
One useful application of Kohlrausch's law is to estimate the limiting equivalent conductivities of weak electrolytes which, as we observed above, cannot be found by extrapolation. Thus for acetic acid CH 3 COOH ("HAc"), we combine the λ 0 values for H 3 O + and CH 3 COO – given in the above table:
Λ 0 HAc = λ 0 H+ + λ 0 Ac–
How fast do ions migrate in solution?
Movement of a migrating ion through the solution is brought about by a force exerted by the applied electric field. This force is proportional to the field strength and to the ionic charge. Calculations of the frictional drag are based on the premise that the ions are spherical (not always true) and the medium is continuous (never true) as opposed to being composed of discrete molecules. Nevertheless, the results generally seem to be realistic enough to be useful.
According to Newton's law, a constant force exerted on a particle will accelerate it, causing it to move faster and faster unless it is restrained by an opposing force. In the case of electrolytic conductance, the opposing force is frictional drag as the ion makes its way through the medium. The magnitude of this force depends on the radius of the ion and its primary hydration shell, and on the viscosity of the solution.
Eventually these two forces come into balance and the ion assumes a constant average velocity which is reflected in the values of λ 0 tabulated in the table above.
The relation between λ 0 and the velocity (known as the ionic mobility μ 0 ) is easily derived, but we will skip the details here, and simply present the results:
Anions are conventionally assigned negative μ 0 values because they move in opposite directions to the cations; the values shown here are absolute values |μ 0 |. Note also that the units are cm/sec per volt/cm, hence the cm 2 term.
As with the limiting conductivities, the trends in the mobilities can be roughly correlated with the charge and size of the ion. (Recall that negative ions tend to be larger than positive ions.)
Cations and anions carry different fractions of the current
In electrolytic conduction, ions having different charge signs move in opposite directions. Conductivity measurements give only the sum of the positive and negative ionic conductivities according to Kohlrausch's law, but they do not reveal how much of the charge is carried by each kind of ion. Unless their mobilities are the same, cations and anions do not contribute equally to the total electric current flowing through the cell.
Recall that an electric current is defined as a flow of electric charges; the current in amperes is the number of coulombs of charge moving through the cell per second. Because ionic solutions contain equal quantities of positive and negative charges, it follows that the current passing through the cell consists of positive charges moving toward the cathode, and negative charges moving toward the anode. But owing to mobility differences, cations and ions do not usually carry identical fractions of the charge.
Transference numbers are often referred to as transport numbers ; either term is acceptable in the context of electrochemistry. The fraction of charge carried by a given kind of ion is known as the transference number \(t_{\pm}\). For a solution of a simple binary salt,
\[ t_+ = \dfrac{\lambda_+}}{\lambda_+ + \lambda_-}\]
\[ t_- = \dfrac{\lambda_-}}{\lambda_+ + \lambda_-}\]
By definition,
\[t_+ + t_– = 1.\]
To help you visualize the effects of non-identical transference numbers, consider a solution of M + X – in which t + = 0.75 and t – = 0.25. Let the cell be divided into three [imaginary] sections as we examine the distribution of cations and anions at three different stages of current flow.
Transference numbers can be determined experimentally by observing the movement of the boundary between electrolyte solutions having an ion in common, such as LiCl and KCl:
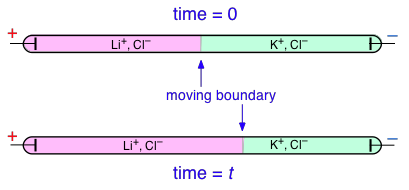
In this example, K + has a higher transference number than Li + , but don't try to understand why the KCl boundary move to the left; the details of how this works are rather complicated and not important for the purposes of this this course.
H + and OH – ions "migrate" without moving, and rapidly!
You may have noticed from the tables above that the hydrogen- and hydroxide ions have extraordinarily high equivalent conductivities and mobilities. This is a consequence of the fact that unlike other ions which need to bump and nudge their way through the network of hydrogen-bonded water molecules, these ions are participants in this network. By simply changing the H 2 O partners they hydrogen-bond with, they can migrate "virtually". In effect, what migrates is the hydrogen-bonds, rather than the physical masses of the ions themselves.
This process is known as the Grothuss Mechanism . The shifting of the hydrogen bonds occurs when the rapid thermal motions of adjacent molecules brings a particular pair into a more favorable configuration for hydrogen bonding within the local molecular network. Bear in mind that what we refer to as "hydrogen ions" H + (aq) are really hydronium ions H 3 O + . It has been proposed that the larger aggregates H 5 O 2 + and H 9 O 4 + are important intermediates in this process.
It is remarkable that this virtual migration process was proposed by Theodor Grotthuss in 1805 — just five years after the discovery of electrolysis, and he didn't even know the correct formula for water; he thought its structure was H–O–O–H.
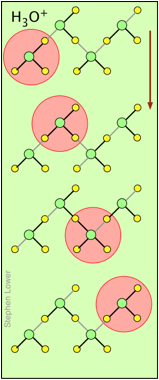
These two diagrams will help you visualize the process. The successive downward rows show the first few "hops" made by the virtual H + and OH – ions as they move in opposite directions toward the appropriate electrodes. (Of course, the same mechanism is operative in the absence of an external electric field, in which case all of the hops will be in random directions.)
Covalent bonds are represented by black lines, and hydrogen bonds by gray lines.
Talk to our experts
1800-120-456-456
- Kohlrausch Law

What is Kohlrausch Law?
Kohlrausch law states that at infinite dilution, when dissociation is complete, each ion makes a definite contribution towards equivalent conductance of the electrolyte irrespective of the nature of the ion with which it is associated and the value of equivalent conductance at infinite dilution for any electrolyte is the sum of the contribution of its constituent ions (cations and anions). Thus, we can say it states that ‘conductivity of ions of an electrolyte at infinite dilution is constant and it does not depend on nature of co-ions.’
\[\lambda_{eq}^{\infty} = \lambda_{c}^{\infty} + \lambda_{a}^{\infty}\]
\[\lambda_{eq}^{\infty}\] = Molar conductivity at infinite dilution
\[\lambda_{C}^{\infty}\]= Conductivity of cation at infinite dilution
\[ \lambda_{a}^{\infty}\]= Conductivity of anion at infinite dilution
When the concentration of the electrolyte is almost zero, at that point, molar conductivity is called limiting molar conductivity.
The molar conductivity of the solution can be defined as the volume of the solution that is conducting that also contains one mole of electrolyte when kept between two electrodes with a unit area of cross-section and one unit length of distance. With the decrease in the concentration, the molar conductivity increases. The increase in the molar conductivity is due to the increase in the volume that comprises one mole of electrolytes. The molar conductivity is known as limiting molar conductivity, Ëm°, when the concentration of the electrolyte approaches zero.
Explanation of Kohlrausch Law
Any random electrolyte is the general case of this law which can be denoted as \[ A_{x}B_{y}\].
Thus mathematically, the limiting molar conductivity of \[ A_{x}B_{y}\] can be represented as:
\[ \lambda _{AxBy}^{\infty} = 2 \lambda_{A + B}^{\infty} + \lambda_{B - x}^{\infty}\]
Where \[\lambda^{\infty}\] is the limiting molar conductivity of the electrolyte chosen.
When a cation is the same in both the electrolytes, then the difference in the molar conductivity of the two electrolytes does not depend upon the cation and is only dependent on the change that happens in their anions. The statement mentioned is also true if the anions are the same and the cations are different.
For instance, if there are two pairs of electrolytes with the same cation A and D in each pair, then the difference between their limiting molar conductivities is not affected by A or D. This can be mathematically represented as,
\[ \lambda _{AB}^{\infty} - \lambda _{AC}^{\infty} = \lambda _{DB}^{\infty} - \lambda _{DC}^{\infty}\]
\[ \lambda _{AB}^{\infty}\] is limiting molar conductivity of AB,
\[\lambda _{AC}^{\infty}\] is limiting molar conductivity of AC,
\[ \lambda _{DB}^{\infty}\] is limiting molar conductivity of DB, and
\[ \lambda _{DC}^{\infty}\] is limiting molar conductivity of DC.
Uses of Kohlrausch’s Law
Kohlrausch’s law is used to calculate molar conductivity at infinite dilution for the weak electrolytes. It’s very difficult or impossible to calculate the molar conductivity of weak electrolytes at infinite dilution. as the conductance of these types of solutions is very low and dissociation of these electrolytes is not completed at high dilutions as well. For example, acetic acid is a weak electrolyte and its molar conductivity at infinite dilution can be calculated by Kohlrausch’s Law. It can be represented as follows:
\[\mu^{\infty}\] = Molar conductance at infinite dilution
\[\mu^{\infty} = \mu^{\infty} = m \lambda _{+}^{\infty} + m \lambda _{-}^{\infty}\]
m and n are a number of ions formed.
For example – molar conductance of aluminium sulphate at infinite dilution can be written as follows –
The formula of aluminium sulphate is \[Al_{2}(SO_{4})_{3}.\]
So, molar conductance at infinite dilution = \[ \mu _{Al_{2}}^{\infty } (SO_{4})_{8} = 2 \lambda _{Al^{s}}^{\infty } + 3\lambda _{SO{_{4}}^{2-}}^{\infty }\]
The knowledge of molar conductivities at infinite dilution of the strong electrolyte like HCl, \[ (CH_{3}COONa)]\, and NaCl and the molar conductivity of acetic acid at infinite dilution can be obtained as follows:
\[ \lambda_{m(HCl)}^{\infty } = \lambda _{H^{+}}^{\infty } + \lambda _{Cl^{-}}^{\infty } \]
\[\lambda_{m(NaCl)}^{\infty } = \lambda _{Na^{+}}^{\infty } + \lambda _{Cl^{-}}^{\infty }\]
\[\lambda_{m(CH_{3}COONa)}^{\infty } = \lambda _{CH_{3}Coo^{-}}^{\infty } + \lambda _{Na^{+}}^{\infty }\]
\[\lambda_{m(CH_{3}COOH)}^{\infty } = \lambda _{CH_{3}Coo^{-}}^{\infty } + \lambda _{H^{+}}^{\infty }\]
\[\lambda_{m(CH_{3}COOH)}^{\infty } = [\lambda _{CH_{3}Coo^{-}}^{\infty } + \lambda _{Na^{+}}^{\infty }] - [ \lambda _{Na^{+}}^{\infty } + \lambda _{Cl^{-}}^{\infty }] [ \lambda _{H^{+}}^{\infty } + \lambda _{Cl^{-}}^{\infty }\]
Determination of the degree of dissociation of the weak electrolytes is given by the following equation:
\[ \alpha = \frac{\lambda _{m}}{ \lambda _{m}^{o}} \]
Determining the dissociation constant (K) of weak electrolytes
\[ K_{a} = \frac{\Lambda _{m}^{2} .C}{\Lambda _{m}^{2}\left [ 1 - \frac{\Lambda _{m}}{\Lambda _{m}^{o}} \right ] } \]
\[ \Rightarrow K_{a} = \frac{\Lambda _{m}^{2}.C}{\Lambda _{m}^{o2}\left ( \frac{\Lambda _{m}^{o} - \Lambda _{m}}{\Lambda _{m}} \right )}\]
\[ \Rightarrow K_{a} = \frac{\Lambda _{m}^{2}C}{\Lambda _{m}^{o}(\Lambda _{m}^{o} - \Lambda _{m})} \]
Thus, the dissociation constant for weak electrolytes at a specific concentration of the solution can be calculated by using the following formula-
\[ K_{c} = \frac {Ca ^{2}} {1 - \alpha} \]
K = dissociation constant,
C = concentration of the solution, and
α = degree of dissociation.
Kohlrausch’s law is used for the calculation of solubility of moderately soluble salt. Some salts that dissolve in very small quantities in water are called moderately or sparingly soluble salts. For example – silver chloride, barium sulphate, lead sulphate, etc.
Acid dissociation constant \[K_{a}\] can also be calculated by Kohlrausch’s law.
When the concentration of the electrolyte is almost zero, at that point, molar conductivity is called limiting molar conductivity. By Kohlrausch’s law, we can determine limiting molar conductivity for an electrolyte.

FAQs on Kohlrausch Law
1. Name the scientist who discovered the law of the independent migration of ions.
From observing experimental data of conductivities of various electrolytes, Friedrich Kohlrausch discovered the law of the independent migration of ions.
2. State the applications of Kohlrausch law of independent migration of ions.
It is used to calculate the limiting molar conductivity, degree of dissociation, and dissociation constant of weak electrolytes. It is also used for calculating the measurement of the solubility of the salt.
NCERT Study Material
If you're seeing this message, it means we're having trouble loading external resources on our website.
If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked.
To log in and use all the features of Khan Academy, please enable JavaScript in your browser.
Physical Chemistry (Essentials) - Class 12
Course: physical chemistry (essentials) - class 12 > unit 1.
- Introduction to electrolysis
- Types of Conductance.
- Variation of conductivity part 1
- Variation of conductivity part 2
- Calculating various types of conductivities.
- Kohlrausch's law of independent migration
Applications of Kohlaursch's law
- Your answer should be
- an integer, like 6
- a simplified proper fraction, like 3 / 5
- a simplified improper fraction, like 7 / 4
- a mixed number, like 1 3 / 4
- an exact decimal, like 0.75
- a multiple of pi, like 12 pi or 2 / 3 pi
In 1874, Kohlrausch formulated the law of independent migration of ions based on the experimental data of conductivities of various electrolytes. This law can be stated as follows:
At infinite dilution, the dissociation of the electrolyte is complete and hence each ion makes definite contribution to the equivalent conductivity of the electrolyte irrespective of the nature of other ions associated with it.
Therefore the limiting equivalent conductivity of an electrolyte is the algebraic sum of limiting equivalent conductivities of its constituent ions.
i.e., The limiting equivalent conductivity of an electrolyte, Λ o electrolyte
Where λ o + and λ o - are the limiting equivalent conductivities of cation and anion respectively.
However the Kohlrausch law can also be stated in terms of molar conductivities as:
The limiting molar conductivity of an electrolyte is the sum of individual contributions of limiting molar conductivities of its constituent ions.
i.e., The molar equivalent conductivity of an electrolyte, μ o electrolyte
Where μ o + and μ o - are the limiting molar conductivities of cation and anion respectively.
And n + and n - are the stoichiometric numbers of positive and negative ions formed during the dissociation of electrolyte.
Kohlrausch observed that at infinite dilutions, the difference between the conductivities of sodium and potassium salts is constant irrespective of the associated anions, as tabulated below.
Kohlrausch argued that the constant difference in the conductivities of above pairs can be ascribed to the fact that the mobility of sodium and potassium ions at infinite dilution is not influenced by the nature of counter ions. The ions at such a low concentration migrate in the electric field as they are independent i.e., they show same ionic conductance irrespective of the nature of counter ion.
1) Calculation of limiting conductivities of weak electrolytes: The Kohlrausch law can be used to calculate the limiting conductivities of weak electrolytes.
E.g., The calculation of limiting equivalent conductance of acetic acid, a weak electrolyte is illustrated below.
According to Kohlrausch law, the limiting equivalent conductance values of CH 3 COOH, CH 3 COONa, HCl and NaCl can be written as follows:
2) Determination of degree of ionization (α) of weak electrolyte: The degree of ionization of a weak electrolyte at a particular concentration is equal to the ratio of actual number of ions formed due to partial ionization to the expected number of ions formed upon complete dissociation.
Since the conductance is proportional to the number of ions in the solution, the degree of ionization is equal to the conductance ratio as given below.
Where
Λ c = equivalent conductivity at given concentration.
Λ o = limiting equivalent conductivity.
λ o + = limiting equivalent conductivity of cation.
λ o - = limiting equivalent conductivity of anion.

Kohlrausch’s law of independent migration of ions: 3 applications
- March 8, 2022
- Electrochemistry
Table of Contents
Kohlrausch’s law of independent migration of ions was given by Kohlrausch in 1875. This law is valid for both strong and weak electrolytes, therefore, it is the most important law. This law is based on the concept that at infinite dilution, electrolytes are completely ionized and in this case, the interionic attraction is very low or it can be assumed that there are no such effects.
Kohlrausch’s law
According to Kohlrausch’s law , the sum of the equivalent ionic conductances of ions of an electrolyte gives the equivalent conductance of that electrolyte at infinite dilution. At infinite dilution, ions are far apart from each other and hence interionic attractions are almost absent. This is the reason for the maximum equivalent conductance of electrolytes at infinite dilution.
This law can be stated in another way ” At infinite dilution where dissociation of all electrolytes is complete and where all interionic attractions disappear, each ion migrates independently of its co-ion(partner ions) and contributes to the total equivalent conductance.
Mathematically, Kohlrausch’s law can be expressed as:
λ ∞ = λ + + λ –
or λ ∞ = λ c + λa
where, λ ∞ = equivalent conductance at infinite dilution
λ + or λ c = equivalent conductance of cation at infinite dilution
λ – or λ a = equivalent conductance of anion at infinite dilution.
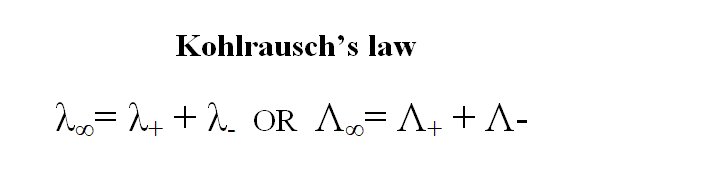
Let us consider an electrolyte AB which dissociates completely at infinite dilution. If Z ohm -1 cm 2 eq -1 be the equivalence conductance of AB at infinite dilution, X and Y ohm -1 cm 2 eq -1 be the equivalent ionic conductances of cation A+ and anion B- respectively, then according to Kohlrausch’s law:
Let’s take the example of NaCl. The equivalent conductance of NaCl at infinite dilution at 25°C is found to be 126.45 ohm -1 cm 2 eq -1 . The equivalent conductance of Na + and Cl – ion is 50.11 and 76.34 respectively, then According to the above law, the sum of equivalent conductance of component ions at infinite dilution must equal that of NaCl.
Application of Kohlrausch’s law
Some major applications of Kohlrausch’s law of independent migration of ions are listed below:
- It can be used to calculate the λ ∞ value of weak electrolytes. Weak electrolytes do not ionizes completely even at great dilution thus practical determination is impossible. Therefore, in such case Kohlrausch’s law is applicable.
- It can be used to determine the solubility of sparingly soluble salts such as AgCl, PbSO 4
- Kohlrausch’s rule is applicable in the determination of degree of ionization of electrolytes and ionic product of water.
- Arun Bahl, B. S. Bahl & G. D. Tuli, Essentials of Physical Chemistry , S. Chand and CompanyLtd., New Delhi, 2012
- M. L. Sharma & P. N. Chaudhary, A Textbook of B. Sc. Chemistry (Volume II), 2nd Edition, Ekta Books Nepal, 2007
Share this to:
- Tags: application of kohlrausch's law , Kohlrausch's law , kohlrausch's law expression , Kohlrausch's law notes
You may also like to read:

Evaporation: Definition, Process, and 5 Reliable Example

Cationic Polymerization: An Easy Mechanism and Kinetics

Corrosion: Definition, Cause, Types, Control, and 7 Importance

Polymorphism and Isomorphism: Definition and 5 Reliable Differences

Bronsted Bjerrum equation and Kinetic salt effect

Diffusion Controlled Reaction: Easy Definition, Kinetics

Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
Top Universities in the USA for Undergraduate and Graduate Chemistry Courses
Preparation of Phthalimide from Phthalic acid by two-step synthesis: Useful Lab Report
Feel Good Hormones: Dopamine, Oxytocin, Serotonin, and Endorphin
Invisible ink: Chemistry, Properties, and 3 Reliable Application
Pyrrole Disorder: Symptoms, Causes, and Treatment
The Chemistry of Mehendi: Composition, Side effects, and Reliable Application
How to Balance Redox Equations Using a Redox Reaction Calculator
Sugar Vs Jaggery: Differences, Calories And Many more
Centrifugation: Definition, Principle, Types, and 3 Reliable Application
Eppendorf Tube: Definition, Types, and Reliable Uses
Litmus Paper: Definition, Chemistry, Test, and 4 important Applications
Our mission is to provide free, world-class Chemisry Notes to Students, anywhere in the world.
Chemist Notes | Chemistry Notes for All | 2024 - Copyright©️ ChemistNotes.com
- Thermodynamics
- Analytical Chemistry
- Atomic Structure
- Classification of Organic Compounds
- Periodic Table of Elements
- Nuclear Force
- Importance of Chemistry
- Chemistry Notes Class 12
- Chemistry Notes Class 8
- Chemistry Notes Class 9
- Chemistry Notes Class 10
- Chemistry Notes Class 11
- Leclanche Cell
- Isoelectric Point
- Electrochemical Cells
- Electrochemistry - Cells and Batteries
- Clemmensen Reduction
- Salt Bridge
- Electrolysis of Molten Salts
- Van Der Waals Force
- Nonelectrolytes - Definition, Meaning, Examples
- F Block Elements
- What is Fructose?
- Azeotropic Mixture
- Halogenation
- Reaction of Esters
- Determination of Boiling Point of Organic Compounds
- Gas Chromatography
- Electrolytic Refining
Kohlrausch Law
Kohlrausch’s law, also known as the law of Independent migration of Ions, tells us that the total of the limiting molar conductance of cations and anions of an electrolyte is equal to the molar conductivity of that electrolyte. This law helps us study electrochemical cells and diluted liquids and is applicable in determining weak electrolytes’ molar conductivity.
In this article, we will learn the concepts of Kohlrausch law, its applications, etc. We have to study Kohlrausch Law in Class 12 for board exams.
Table of Content
What is Kohlrausch’s Law?
What is molar conductivity, formula of kohlrausch law, kohlrausch’s law of independent migration, application of kohlrausch’s law.
Kohlrausch Law, also known as Kohlrausch’s Law of Independent Migration of Ions, refers to an electrolyte’s limiting molar conductivity to its constituent ions. The law was proposed by Friedrich Kohlrausch in the late 19th century and is used to calculate the limiting molar conductivity, degree of dissociation, and dissociation constant of weak electrolytes. It is also used to measure the solubility of the salt.
For example, the limiting molar conductivity, of sodium chloride is determined when the limiting molar conductivities of sodium ion and chloride ion are given.
Λ o NaCl = Λ o Na+ + Λ o Cl-
Statement of Kohlrausch law
Limiting molar conductivity of an Electrolyte equals the sum of the individual limiting molar conductivities of the cations and anions that make up the electrolyte.
Molar conductivity is a measure of the ability of a substance to conduct electricity in solution. It is defined as the conductivity of a solution containing one mole of the substance in question. Molar conductivity is usually denoted by the symbol Λ m and is expressed in units of Siemens per meter squared per mole (S m² mol⁻¹).
The molar conductivity of a solution increases with the decrease in concentration. This increase in molar conductivity is because of the rise in the total volume containing one mole of the electrolyte. When the electrolyte concentration approaches zero, the molar conductivity is known as the limiting molar conductivity, Λ° m . Kohlrausch’s Law is based on molar conductivity and is widely used to study dilute liquids and electrolyte solutions.
Kohlrausch’s Law, also known as the Law of Independent Migration of Ions, states that at infinite dilution when dissociation is complete, each ion makes a definite contribution towards the equivalent conductance of the electrolyte. In other words, the equivalent conductivity of an electrolyte at infinite dilution is equal to the sum of the conductances of the individual ions. The law can be expressed as:
λ ∞ eq = λ ∞ c + λ ∞ a where, λ eq ∞ is Molar Conductivity at infinite dilution λ c ∞ is Conductivity of the cation at infinite dilution λ a ∞ is Conductivity of the anion at infinite dilution
Kohlrausch’s Law is essential for calculating the limiting molar conductivity, degree of dissociation, and dissociation constant of weak electrolytes, as well as for determining the solubility of salts.
It is based on the concept that at infinite dilution when dissociation is complete, each ion makes a definite contribution towards the equivalent conductance of the electrolyte. The law is beneficial in studying dilute liquids and electrolyte solutions, as it allows for calculating the behavior of ions in such systems.
Some applications of Kohlrausch’s law of independent migration of ions are:
Kohlrausch’s Law of Independent Migration of Ions can be represented graphically by plotting an electrolyte’s molar conductivity (λm) against the square root of its concentration (√c). According to Kohlrausch’s Law, the plot should be a straight line with an intercept equal to the limiting molar conductivity (λ∞m) and a slope similar to -A, where A is a constant.
The value of A depends on the type of electrolyte and the temperature at which the measurement is taken. The graph obtained by plotting λm against √c helps determine the limiting molar conductivity of weak electrolytes, which cannot be obtained by extrapolation of molar conductivity to zero concentration. The value of λ∞m can be determined from the intercept of the straight line obtained from the plot. The graph can also be used to calculate the degree of dissociation and dissociation constant of weak electrolytes.

At lower concentrations, weak electrolytes exhibit a steep increase in molar conductivity. Therefore, extrapolating molar conductivity to zero concentration will not yield Λ, the limiting molar conductivity. Consequently, we calculate the limiting molar conductivity for weak electrolytes, Λ, using the Kohlrausch law of independent ion migration.
For a weak electrolyte at a particular concentration, Kohlrausch’s law also aids in calculating the dissociation constant from the molar conductivity and limiting molar conductivity values.
α = Λ/Em° where, α is Dissociation Constant Λ is Molar Conductivity Em° is Limiting Molar Conductivity
Some of the critical uses of Kohlrausch’s Law include:
- Calculation of the dissociation constant of an electrolyte: Kohlrausch’s Law can be used to determine the dissociation constant of an electrolyte by studying the molar conductivity at infinite dilution.
- Calculating the limiting molar conductivity of a weak electrolyte: The law allows for estimating the limiting molar conductivity of weak electrolytes, which is essential for understanding their behavior in dilute solutions.
- Determination of the degree of dissociation of weak electrolytes: Kohlrausch’s Law can be used to determine the degree of dissociation of weak electrolytes by studying their molar conductivity at different concentrations.
- Calculation of the solubility of sparingly soluble salts: The law can be used to calculate the solubility of sparingly soluble salts by determining their limiting molar conductivity.
- Determination of the transport number of ions in an electrolyte: Kohlrausch’s Law can be used to calculate the transport number of ions in an electrolyte, which is an essential parameter in electrochemical systems.
- Determination of the ionic strength of a solution: Kohlrausch’s Law can be used to determine the ionic strength of a solution, which is a crucial parameter in electrochemical systems.
Equivalent Conductance Formula Electrochemical Cells Primary and Secondary Cells
Kohlrausch Law Class 12 – Solved Problems
Q1. The equivalent conductance of a strong electrolyte increases on dilution due to
- An increase in the number of ions and the ionic mobility of the solution
- Complete dilution of the electrolyte at standard dilution
- An increase in the ionic mobility of solution
- None of Above
Option (3) is Correct
Explanation:
Equivalent conductance of a strong electrolyte increases on dilution due to an increase in the ionic mobility of the solution.
Q2. Calculate the limiting molar conductivity of sodium sulphate(Na 2 SO 4 ). If the limiting molar conductivity of Na + is 50.1 S.cm 2 /mol and 1/2 SO 4 2- is 80.0 S.cm 2 /mol.
Given, λ ∞ Na+ = 50.1 S.cm 2 /mol 1/2 λ ∞ SO42− = 80.0 S.cm 2 /mol According to Kohlrausch’s Law of Independent Migration λ ∞ (Na 2 SO 4 ) = 2λ ∞ Na+ + 2×(1/2λ ∞ SO42− ) λ ∞ (Na 2 SO 4 ) = 2 (50.1)+2×80 => λ ∞ (Na 2 SO 4 ) = 260.2 S.cm 2 /mol So, the limiting molar conductivity of sodium sulphate is 260.2 S.cm 2 /mol.
Q3. The molar conductance of a solution ____ with dilution while its specific conductance ___ with dilution.
- Decreases, Increases
- Increases, Decreases
- Decreases, Decreases
- Increases, Increases
Option (1) is Correct
Molar conductance of a solution increases with dilution while its specific conductance decreases with dilution.
Kohlrausch’s Law – FAQs
1. define kohlrausch law.
Kohlrausch law, named after Friedrich Kohlrausch, describes the conductivity of electrolyte solutions and states that each ion in a solution contributes independently to the overall conductivity.
2. What is Mathematical Expression of Kohlrausch Law?
The mathematical expression of Kohlrausch Law is: Λ m = λ + c + + λ − c −
3. What is Infinite Dilution in Electrochemistry?
Infinite dilution refers to the state when the concentration of an electrolyte becomes extremely low, approaching zero. In this condition, ions are widely separated, allowing for precise measurement of ion conductivities.
4. What is Kohlrausch Law and its Applications?
Kohlrausch’s law helps predict and understand the conductivity of electrolyte solutions. Its applications include determining molar conductivities and ion mobility and studying the dissociation of electrolytes in various concentrations.
5. Who Discovered Law of Independent Migration of Ions?
The law of independent migration of ions was discovered by Friedrich Kohlrausch in the late 19th century, contributing significantly to our understanding of electrolyte behavior.
Please Login to comment...
Similar reads.
- Chemistry-Class-12
- Electrochemistry
- School Chemistry
- School Learning
- 10 Best Todoist Alternatives in 2024 (Free)
- How to Get Spotify Premium Free Forever on iOS/Android
- Yahoo Acquires Instagram Co-Founders' AI News Platform Artifact
- OpenAI Introduces DALL-E Editor Interface
- Top 10 R Project Ideas for Beginners in 2024
Improve your Coding Skills with Practice
What kind of Experience do you want to share?
- Write for us
The Kohlrausch Law and its Applications
The Kohlrausch Law, also known as Kohlrausch’s Law of Independent Migration of Ions, is a principle in electrochemistry that describes how the molar conductivity of an electrolyte can be calculated as a sum of contributions from individual ions. This law was formulated by the German physicist Friedrich Kohlrausch in the late 19th century.
Definition:
The Kohlrausch Law states that the molar conductivity (Λm) of an electrolyte can be calculated by adding the molar conductivities of its constituent ions, each multiplied by their respective concentration (in moles per unit volume).
Mathematical Formula:
The Kohlrausch Law can be expressed mathematically as follows:
Λm = Λ 0 m-Kc 1/2
- Λm is the molar conductivity of the electrolyte.
- Λ 0 m is the limiting moral conductivity
- K is a coefficient related to stoichiometry of the electrolyte and
- c is the concentration of the electrolyte.
Understanding of electrolyte conductance was rapidly developing. His work significantly contributed to the field of electrochemistry and helped establish the concept that ions in solution contribute independently to the overall conductivity of the electrolyte.
Applications and Uses of the Kohlrausch Law
1. Determination of Ionic Conductivity: The Kohlrausch Law is primarily used to calculate the molar conductivity of an electrolyte solution by considering the contributions of individual ions. This information is essential in understanding the conductance of electrolytes in various applications, such as batteries, fuel cells, and electrochemical cells.
2. Ionic Mobility: The law is instrumental in determining the mobility of individual ions in solution. This information is crucial in studies related to ion transport, diffusion, and migration in electrolytes.
3. Electrolytic Conductance: It plays a significant role in understanding the behavior of electrolytes in various chemical and industrial processes, including chemical synthesis, electroplating, and wastewater treatment.
4.Electrolyte Characterization: It helps in characterizing the behavior of different electrolytes. Strong electrolytes (those that dissociate completely in solution) and weak electrolytes (those that partially dissociate) can be distinguished and their behavior is explained using this law.
5. Dilution Effects: The law provides insights into how the conductivity of an electrolyte changes with dilution. It helps in understanding the behavior of electrolyte solutions at different concentrations.
6. Quality Control and Analysis : In various industries, especially in the pharmaceutical and chemical sectors, the law is used for quality control and analysis of solutions and materials that involve ionic compounds. It can help ensure that the correct ions are present in the desired concentration.
7. Electrochemical Cells and Batteries: Understanding the behavior of electrolytes is crucial in the design and operation of electrochemical cells and batteries. Kohlrausch’s law can be used to predict and optimize the performance of such systems.
8. Environmental Monitoring: The law is used in environmental science to analyze the conductance of water samples, which can provide information about the presence of ions and pollutants in natural water bodies.
9. Teaching and Research: It is a fundamental concept in the field of physical chemistry and is taught in academic settings to help students understand the behavior of electrolyte solutions. It is also used in research to analyze and model the behavior of various electrolytes.
10. pH and Acid-Base Equilibria : Kohlrausch’s law is used in understanding the behavior of strong and weak acids and bases and their conductance in solution, which is important in studying pH and acid-base equilibria.
Let’s consider a simple example involving the electrolyte sodium chloride (NaCl) dissolved in water:
- NaCl dissociates into two ions: Na+ and Cl-
- Suppose we have a 0.1 M NaCl solution.
- The molar conductivity (Λm) of NaCl can be calculated using the Kohlrausch Law as follows:
Λm(NaCl) = (Λ(Na+) * [Na+]) + (Λ(Cl-) * [Cl-])
Λ(Na+) and Λ(Cl-) are the molar conductivities of Na+ and Cl-, respectively. The molar conductivities of individual ions are typically measured experimentally and can be found in reference tables.
By plugging in the values for Λ(Na+) and Λ(Cl-) and the concentrations of Na+ and Cl-, you can calculate the molar conductivity of the NaCl solution.
Conclusion:
The Kohlrausch Law is an essential tool in electrochemistry and plays a crucial role in understanding and quantifying the conductance of electrolytes in various chemical and industrial processes.
Leave a Reply Cancel Reply
Save my name, email, and website in this browser for the next time I comment.
Automated page speed optimizations for fast site performance
- Bihar Board
SRM University
Ap inter results.
- AP Board Results 2024
- UP Board Result 2024
- CBSE Board Result 2024
- MP Board Result 2024
- Rajasthan Board Result 2024
- Shiv Khera Special
- Education News
- Web Stories
- Current Affairs
- नए भारत का नया उत्तर प्रदेश
- School & Boards
- College Admission
- Govt Jobs Alert & Prep
- GK & Aptitude
- Chemistry Study Material
Kohlrausch’s Law: Definition, Formula and Applications, Download PDF Here!
kohlrausch law chemistry: know easy explanations for the definition, formula and applications of kohlrausch’s law here for a quick understanding of this important chemistry concept. download pdf for revision of kohlrausch’s law as and when required..

What is Kohlrausch ’s Law?
Definition:, formula for kohlrausch's law.
The mathematical formula for Kohlrausch's law is as follows:
Λ ∞ = Λ ∞ + + Λ ∞ −
Λ ∞ = Equivalent conductivity of the electrolyte at infinite dilution
Λ ∞ + = Equivalent conductivity of the cation at infinite dilution
Applications
- Determining degree of dissociation: The degree of dissociation of an electrolyte is the fraction of the electrolyte molecules that have dissociated into ions. Kohlrausch's law can be used to determine the degree of dissociation of an electrolyte by measuring its equivalent conductivity at different concentrations.
- Calculating limiting molar conductivity: The limiting molar conductivity of an electrolyte is the equivalent conductivity of that electrolyte at infinite dilution. It can be computed by adding the limiting molar conductivities of the individual ions in the electrolyte.
- Determining solubility of sparingly soluble salts: Kohlrausch's law can be used to determine the solubility of sparingly soluble salts by measuring the equivalent conductivity of the salt solution.
- Determining d issociation constant for weak electrolytes : Kohlrausch's law can be used to determine the dissociation constant for a weak electrolyte by measuring the molar conductivity or specific conductivity of the electrolyte at different concentrations.
Get here latest School , CBSE and Govt Jobs notification in English and Hindi for Sarkari Naukari and Sarkari Result . Download the Jagran Josh Sarkari Naukri App . Check Board Result 2024 for Class 10 and Class 12 like CBSE Board Result , UP Board Result , Bihar Board Result , MP Board Result , Rajasthan Board Result and Other States Boards.
- NDA Admit Card 2024
- TNPSC Group 1 Hall Ticket 2024
- APPSC Group 1 Result 2024
- AIASL Executive Recruitment 2024
- NTA NITTT Result 2024
- APPSC Group 2 Result 2024
- CUET PG Answer Key 2024
- TN SET Application Form 2024
- BPSC Head Master 2024 Last Date Extended
- UGC NET Notification 2024
Latest Education News
Find 3 differences between the man drinking red wine pictures in 13 seconds!
CUET UG 2024 Correction Window Close Tomorrow, Exam City Slip On April 30
HPSC Veterinary Surgeon Question Paper 2024: डाउनलोड करें सेट A, B, C और D पेपर PDF
HPSC Veterinary Surgeon 2024 Question Paper: Download PDF Here
Find 3 differences between the rugby player pictures in 17 seconds!
HPSC AEE Question Paper 2024: Download PDF Here
दुनिया के 10 सबसे बड़े नेशनल पार्क की सूची, पढ़ें
IPL Points Table 2024: आईपीएल 2024 अपडेटेड पॉइंट टेबल यहां देखें
आईपीएल 2024 में 17 मैचों में ही टूटा छक्कों का रिकॉर्ड, अब तक लगे इतने छक्के
Surya Grahan 2024: मोबाइल पर ऐसे देखें साल के पहले सूर्यग्रहण की LIVE स्ट्रीमिंग, डायरेक्ट लिंक यहां देखें
Optical Illusion Vision Test: Find the cat in the picture in 3 seconds!
कैसे हुआ था महासागरों का निर्माण, जानें
उत्तर प्रदेश में कुल कितने मंडल हैं, जानें
MH SET Cut Off 2024 Marks: Check Minimum Qualifying Marks & Previous Year Cut Off
भारत के 10 सबसे पुराने रेलवे स्टेशन कौन-से हैं, जानें
WB Police SI 2024 Registration Last Date Today, Direct Apply Online Link Here
Find 3 differences between the angel and devil pictures in 12 seconds!
NVS Non Teaching Recruitment 2024: Notification Out For 1377 Posts, Apply Till Apr 30
Rajju Bhaiya University Result 2024 OUT at prsuniv.ac.in; Direct Link to Download UG and PG Marksheet PDF
Picture Puzzle IQ Test: Find the mistake in the picture in 7 seconds!
Write Kohlrausch law and give one application of it :
Kohlrausch law of independent migration of ions: the law states that at infinite dilution, each ion migrates independently of its co-ion and make its own contribution to the total molar conductivity of an electrolyte irrespective of the nature of other ion with which it is associated. the molar conductivity of the electrolyte at zero concentration is equal to the sum of molar conductivity of cation and the molar conductivity of anion at infinite dilution. Λ 0 = λ 0 + + λ 0 − application: the law is used to calculate the molar conductivity of any electrolyte at zero concentration. for example, the molar conductivity of acetic acid at zero concentration can be calculated from the following expression. Λ 0 ( c h 3 c o o h ) = Λ 0 ( h c l ) + Λ 0 ( c h 3 c o o n a ) − Λ 0 ( n a c l ).
What is Kohlrauch law . Give it's main application s ?
- Chemistry Concept Questions and Answers
- Kohlrausch law Questions
Kohlrausch Law Questions
Kohlrausch Law directs the electrolyte’s limiting molar conductivity with its constituent ions. It displays that at infinite dilution equivalent conductivity of an electrolyte is equivalent to the sum of the conductances of the cations and anions. For example, the equivalent conductivity of CH 3 COONa at equivalent dilution would be equivalent to the sum of the conductances of the acetate and sodium ion.
𝛬 m º CH 3 COONa = 𝛬 m º CH 3 COO – + 𝛬 m º Na +
Kohlrausch Law Chemistry Questions with Solutions
Q1. Equivalent conductance of a strong electrolyte increases on dilution due to
(a) An increase in the number of ions and the ionic mobility of solution
(b) Complete dilution of the electrolyte at standard dilution
(c ) An increase in the ionic mobility of solution
(d) None of the above
Answer: (c ) Equivalent conductance of a strong electrolyte increases on dilution due to an increase in the ionic mobility of solution.
Q2. The molar conductivity of an ionic solution depends on
(a) Concentration of electrolytes in solution
(b) Distance between electrodes
(c ) Surface area of electrodes
Answer: (a) The molar conductivity of an ionic solution depends on the concentration of electrolytes in solution.
Q3. The molar conductance of a solution ___________ with dilution while its specific conductance ________ with dilution.
(a) Decreases, Increases
(b) Increases, Decreases
(c ) Decreases, Decreases
(d) Increases, Increases
Answer: (a) The molar conductance of a solution increases with dilution while its specific conductance decreases with dilution.
Q4. Kohlrausch’s law states that at
(a) Infinite dilution, the equivalent conductivity of an electrolyte is equivalent to the sum of the conductances of the cations and anions
(b) Finite dilution, the equivalent conductivity of an electrolyte is equivalent to the sum of the conductances of the cations and anions.
(c ) Both (a) and (b)
Answer: (a) Kohlrausch’s law states that at infinite dilution, the equivalent conductivity of an electrolyte is equivalent to the sum of the conductances of the cations and the anions
Q5. Which of the following statements is correct for an electrolytic solution upon dilution?
(a) Conductivity increase on dilution
(b) Conductivity decrease on dilution
(c ) Molar conductance decreases, but equivalent conductance increases on dilution
(d) Molar conductance increases, but equivalent conductance decreases on dilution
Answer: (b) When an electrolytic solution is diluted, it’s concentration decreases. Due to this, the conductance increases, and the conductivity decreases.
Q6. What is Kohlrausch’s law?
Answer: Kohlrausch’s law states that at infinite dilution equivalent conductivity of an electrolyte is equivalent to the sum of the conductances of the cations and anions.
Q7. What are the applications of Kohlrausch’s law?
Answer: Kohlrausch’s law states that at infinite dilution equivalent conductivity of an electrolyte is equivalent to the sum of the conductances of the cations and anions. There are a lot of applications of Kohlrausch’s law.
A few of them are mentioned below.
1. It can be used to calculate the molar conductance at infinite dilution for a weak electrolyte.
2. It can be used to calculate the degree of dissociation at infinite dilution for a weak electrolyte.
3. It can be used to calculate the dissociation constant at infinite dilution for a weak electrolyte.
4. It can be used to calculate the solubility of the sparingly soluble salt.
Q8. The 𝛬 m º for sodium iodide, sodium acetate, and magnesium acetate solution are 12.69, 9.10 and 18.78 S cm 2 mol -1, respectively, at 298 K. Calculate 𝛬 m º for magnesium iodide.
We know that
𝛬 m º (NaI) = 12.69 S cm 2 mol -1
𝛬 m º (CH 3 COONa) = 9.10 S cm 2 mol -1
𝛬 m º (MgI 2 ) = 18.78 S cm 2 mol -1
According to Kohlrausch’s law
𝛬 m º (MgI 2 ) = 𝛬 m º [CH 3 (COO)] 2 Mg + 2 𝛬 m º (NaI) – 2 𝛬 m º (CH 3 COONa)
𝛬 m º (MgI 2 ) = (18.78 + 2 X 12.69 – 2 X 9.10) S cm 2 mol -1
𝛬 m º (MgI 2 ) = (18.78 + 25.38 – 18.20) S cm 2 mol -1
𝛬 m º (MgI 2 ) = (44.16 – 18.20) S cm 2 mol -1
𝛬 m º (MgI 2 ) = 25.96 S cm 2 mol -1
Q9. Calculate 𝛬 m º for CaCl 2 and MgSO 4 from the following data:
𝛬 m º (Ca 2+ ) = 119.0 S cm 2 mol -1 , 𝛬 m º (Mg 2+ ) = 106.0 S cm 2 mol -1 , 𝛬 m º (Cl – ) = 76.3 S cm 2 mol -1 and 𝛬 m º (SO 4 2- ) = 160.05 S cm 2 mol -1
We know that,
𝛬 m º (Ca 2+ ) = 119.0 S cm 2 mol -1
𝛬 m º (Mg 2+ ) = 106.0 S cm 2 mol -1
𝛬 m º (Cl – ) = 76.3 S cm 2 mol -1
𝛬 m º (SO 4 2- ) = 160.05 S cm 2 mol -1
𝛬 m º (CaCl 2 ) = [119.0 + 2 X 76.3] S cm 2 mol -1
𝛬 m º (CaCl 2 ) = [119.0 + 152.6] S cm 2 mol -1
𝛬 m º (CaCl 2 ) = 271.6 S cm 2 mol -1
𝛬 m º (MgSO 4 ) = 𝛬 m º (Mg 2+ ) + 𝛬 m º (SO 4 2- )
𝛬 m º (MgSO 4 ) = [106.0 + 160.05] S cm 2 mol -1
𝛬 m º (MgSO 4 ) = 266.05 S cm 2 mol -1
Q10. The 𝛬 m º for sodium acetate, HCl, and NaCl are 91.0, 425.9 and 126.4 S cm 2 mol -1, respectively, at 298 K. Calculate 𝛬 m º for CH 3 COOH.
𝛬 m º (CH 3 COONa) = 91.0 S cm 2 mol -1
𝛬 m º (HCl) = 425.9 S cm 2 mol -1
𝛬 m º (NaCl) = 126.4 S cm 2 mol -1
𝛬 m º CH 3 COOH = 𝛬 m º CH 3 COONa + 𝛬 m º HCl – 𝛬 m º NaCl
𝛬 m º CH 3 COOH = (91.0 + 425.9 – 126.4) S cm 2 mol -1
𝛬 m º CH 3 COOH = 390.5 S cm 2 mol -1
Q11. The molar conductivity of a 1.5 M solution of an electrolyte is found to be 138.9 S cm 2 mol −1 . Calculate the conductivity of this solution.
Answer: Molar conductivity (𝜆 m ) = 138.9 S cm 2 mol −1
Concentration = 1.5 M
Molar conductivity = Conductivity / Concentration
Conductivity = Molar conductivity X Concentration
Conductivity = (138.9 S cm 2 mol −1 X 1.5 M) / 1000 cm 3 L -1
Conductivity = 0.208 S cm -1
Q12. The resistance and conductivity of a cell containing 0.001 M KCl solution at 298 K are 1500 Ω and 1.46 X 10 −4 S cm −1 , respectively. What is the cell constant of the cell?
Resistance = 1500 Ω
Conductivity = 1.46 X 10 −4 S cm −1
Concentration = 0.001 M
Temperature = 298 K
Conductivity = Cell constant / Resistance
1.46 X 10 −4 S cm −1 = Cell constant / 1500 Ω
Cell constant = 1.46 X 10 −4 S cm −1 X 1500 Ω
Cell constant = 0.219 cm -1
Q13. The conductivity of the 0.20 M solution of KCl at 298 K is 0.0248 S cm −1. Calculate its molar conductivity.
Conductivity = 0.0248 S cm −1
Concentration = 0.20 M
Molar conductivity (𝜆 m ) = (Conductivity X 1000) / C
Molar conductivity (𝜆 m ) = (0.0248 S cm −1 X 1000) / 0.20 mol cm -3
Molar conductivity (𝜆 m ) = 24.8 S cm −1 / 0.20 mol cm -3
Molar conductivity (𝜆 m ) = 124 S cm 2 mol −1
Q14. The molar conductivity of NaCl, HCl and CH 3 COONa at infinite dilution are 126.45, 426.16 and 91 S cm 2 mol −1 , respectively. What will be the molar conductivity of CH 3 COOH at infinite dilution?
𝛬 m º (NaCl) = 126.45 S cm 2 mol −1
𝛬 m º (HCl) = 426.16 S cm 2 mol −1
𝛬 m º (CH 3 COONa) = 91 S cm 2 mol −1
𝛬 m º CH 3 COOH = (91 + 426.16 – 126.45) S cm 2 mol −1
𝛬 m º CH 3 COOH = (517.16 – 126.45) S cm 2 mol −1
𝛬 m º CH 3 COOH = 390.71 S cm 2 mol −1
Q15. Match the following.
Practise Questions on Kohlrausch Law
Q1. Why can 𝛬 m º for CH 3 COOH not be determined experimentally?
Q2. Why does the conductivity of a solution decrease with dilution?
Q3. The conductivity of 0.00241 M acetic acid is 7.896 X 10 -5 S cm -1. Calculate its molar conductivity, and if 𝛬 m º for acetic acid is 390.5 S cm 2 mol -1, what is its dissociation constant?
Q4. Three electrolytic cells A, B, and C, containing a solution of ZnSO 4 , AgNO 3 and CuSO 4 , respectively all connected in series. A steady current of 1.5 amperes was passed through then until 1.45 g of silver was deposited at the cathode of cell B. How long did the current flow? What mass of copper and zinc were deposited?
Q5. Suggest a way to determine the 𝛬 m º of CH 3 COOH.
Click the PDF to check the answers for Practice Questions. Download PDF
Recommended Videos
Electrochemistry class 12 chemistry (chapter-3).

JEE Electrochemistry

ElectroChemistry Class 12 Chemistry (Ch-3)

JEE Main 2022 – Top 10 Most Important & Expected Questions of Electrochemistry

- Share Share
Register with BYJU'S & Download Free PDFs
Register with byju's & watch live videos.


IMAGES
VIDEO
COMMENTS
Write applications of Kohlrausch's Law. Advertisements. Solution Show Solution. The theory can be used to calculate the molar conductivity of an electrolyte at the zero concentration. Determination of molar conductivity of weak electrolyte at zero concentration.
Some important applications of Kohlrausch law of independent migration of ions are: Kohlrausch law helps us in the determination of limiting molar conductivities for any electrolyte. Weak electrolytes have lower molar conductivities and lower degree of dissociation at higher concentrations. The graph plotted between molar conductivity and c 1/2 ...
Ans: Kohlrausch's law states that: Molar conductivity of an electrolyte at infinite dilution is the sum of ionic conductivities of each ion (cations and anions) present, multiplied by the number of each ion present in one unit of the electrolyte. The two types of application of Kohlrauch law include: a.
Kohlrausch law and its applications are very important in the study of dilute solutions and also in the study of electrochemical cells. This law is mainly used to find the limiting conductivity of a weak electrolyte, among other important applications. An example of this law is the limiting conductivity of sulphuric acid (H 2 SO 4 ).
Kohlrausch's law is useful in the process of determining an electrolyte's dissociation constant. Calculating the limiting molar conductivity of a weak electrolyte requires the use of this formula. This rule may also be useful to calculate the degrees of dissociation of electrolytes with a low charge. This rule is also useful to compute the ...
Kohlrausch's law greatly simplifies estimates of Λ 0. This principle is known as Kohlrausch's law of independent migration, which states that in the limit of infinite dilution,. Each ionic species makes a contribution to the conductivity of the solution that depends only on the nature of that particular ion, and is independent of the other ions present.
Thus, the dissociation constant for weak electrolytes at a specific concentration of the solution can be calculated by using the following formula-. Kc = Ca2 1 − α. Where. K = dissociation constant, C = concentration of the solution, and. α = degree of dissociation. Kohlrausch's law is used for the calculation of solubility of moderately ...
Kohlrausch's law of independent migration. In this video, we will discuss why it is experimentally challenging to find out the limiting molar conductivity for weak electrolytes. We will learn about Kohlrausch's law and how it can be used for weak electrolytes. Timestamps 00:34 - Recap of how to find limiting molar conductivity for a strong ...
Applications of Kohlaursch's law. Google Classroom. The molar conductivity of 0.02 M CH A 3 COOH is 40 S cm A 2 / mol . Calculate its degree of dissociation ( α ). Given: λ H A + o = 349.6 S cm A 2 / mol. λ CH A 3 COO A − o = 40.9 S cm A 2 / mol . Stuck?
126.50. NaBr. 111.10. 21.20. KBr. 132.30. Kohlrausch argued that the constant difference in the conductivities of above pairs can be ascribed to the fact that the mobility of sodium and potassium ions at infinite dilution is not influenced by the nature of counter ions. The ions at such a low concentration migrate in the electric field as they ...
Some major applications of Kohlrausch's law of independent migration of ions are listed below: It can be used to calculate the λ ∞ value of weak electrolytes. Weak electrolytes do not ionizes completely even at great dilution thus practical determination is impossible. Therefore, in such case Kohlrausch's law is applicable. Kohlrausch ...
Kohlrausch's law has simplified the understanding of how electrolytes transfer electricity through the movement of ions. By using Kohlrausch law, important properties of electrolytes can be easily uncovered and understood. The application of Kohlrausch law is widespread and covers cells, salts, etc.
Kohlrausch Law. Kohlrausch's law, also known as the law of Independent migration of Ions, tells us that the total of the limiting molar conductance of cations and anions of an electrolyte is equal to the molar conductivity of that electrolyte. This law helps us study electrochemical cells and diluted liquids and is applicable in determining ...
Verified by Toppr. Kohlrausch's law : The value of molar conductance at infinite dilution is give by the sum of the contributions of ions (Cation and anion) λ∞ m =xλ∞ +a +yλ∞ −e. λ+2 and λ−e ionic conductance of cation and anion x and y = no. of ions. Two applications. (i) Calculation of molar conductance at infinite dilution for ...
Explanation of Kohlrausch Law. [Click Here for Previous Year's Questions] This law applies to any random electrolyte, which can be denoted as follows: AxBy. Thus mathematically, the limiting molar conductivity of AxBy can be represented as. λ∞ AxBy λ A x B y ∞ = 2λ∞ A+B λ A + B ∞ + λ∞ B−x λ B − x ∞. Where,
Application. Kohlrausch's law has a variety of applications. Formula for determining the degree of dissociation. Calculation of the solubility of a salt that is only sparingly solubleCalculation of the Dissociation Constant for Electrolytes with Low Dissociation Constant. Formula for calculating the molar conductivity of weak electrolytes ...
method based upon Kohlrausch law. Explanation for the variation : The variation of molar conductance with concentration can be explained on the basis of conducting ability of ions for weak and strong electrolytes. For weak electrolytes the variation of with dilution can be explained on the bases of number of ions in solution.
The Kohlrausch Law and its Applications. October 11, 2023. The Kohlrausch Law, also known as Kohlrausch's Law of Independent Migration of Ions, is a principle in electrochemistry that describes how the molar conductivity of an electrolyte can be calculated as a sum of contributions from individual ions. This law was formulated by the German ...
APPLICATIONS OF KOHLRAUSCH LAW 1) Calculation of limiting conductivities of weak electrolytes: The Kohlrausch law can be used to calculate the limiting conductivities of weak electrolytes. E.g., The calculation of limiting equivalent conductance of acetic acid, a weak electrolyte is illustrated below.
Kohlrausch Law: Check definition, formula and applications of Kohlrausch's Law explained in simple and easy to understand format. Download PDF.
Λ0 =λ0 + +λ0 −. Application: The law is used to calculate the molar conductivity of any electrolyte at zero concentration. For example, the molar conductivity of acetic acid at zero concentration can be calculated from the following expression. Λ0(CH 3COOH) = Λ0(H Cl)+Λ0(CH 3COON a)−Λ0(N aCl)
What are the applications of Kohlrausch's law? Answer: Kohlrausch's law states that at infinite dilution equivalent conductivity of an electrolyte is equivalent to the sum of the conductances of the cations and anions. There are a lot of applications of Kohlrausch's law. A few of them are mentioned below. 1.
To develop next-generation lithium-ion batteries with enhanced stability and safety, it is crucial to understand the physicochemical principles of nonaqueous electrolytes. Kohlrausch's law describes a linear decrease in the molar conductivity (Λ) with respect to the square root of the molarity of strong electrolytes at lower concentrations. This empirical law explains the impeded ionic ...