Thesis Helpers
Find the best tips and advice to improve your writing. Or, have a top expert write your paper.

170 Vaccination Research Paper Topics For Stellar Students

Research papers are a monumental highlight in your academic journey. They are a critical milestone in your studies that must be tackled with the utmost care and stellar diligence. Vaccination topics are susceptible as you have to show complete mastery of all details.
If you are pursuing a medicine course, then vaccination research topics might be an excellent area of interest. A good research paper starts with a great topic, and we are here to help you nail that. We understand the significance of research papers, and that is why we have handpicked 170 out-of-the-box vaccination research paper topics, titles, and ideas to make your work seamless.
Debate Topics About Vaccination
- What is reverse vaccinology?
- Look at the ways of harnessing the participation of dendritic cells in tolerance and immunity
- What are some of the approaches to advance cancer vaccines to clinical utility?
- Highlight innovative therapeutic and vaccine approaches against respiratory pathogens
- Examine immunity to malaria and vaccine strategies
- Assess molecular vaccines against pathogens in the post-genomic era
- Comprehending the limitations of today’s influenza vaccine strategies and further development of more efficient therapeutic and preventative interventions
- Study HIV-associated persistent inflammation and immune activation
- Analyze recent advances in respiratory virus infection
- What is the novel approach for anti-tumor vaccines
- Unravel the challenges and progress in the development of a B cell-based hepatitis C virus vaccine
- What is the functional relevance of Tatraspanins in the immune system?
- Look at advanced immunization technologies for next-generation vaccines
- Evaluate epitope discovery and synthetic vaccine design
- In what ways can tuberculosis be treated by targeting host immunity
- What are the immunomodulatory effects of drugs in the treatment of immune-related diseases
- Highlight natural antibodies in health and disease
- Discuss different influenza virus vaccines and immunotherapy
- What are some of the shadows of cancer immunotherapy
- Understanding the therapeutical potential of extracellular vesicles
- A review of the ethical theories and problems associated with vaccination in America
- Do vaccines love the Darwinian fitness of immune cells
Vaccination Behavior Research Topics
- Unraveling demand and supply effects on the up-take of influenza vaccinations
- Point out new approaches to the seasonal flu vaccine
- Exploring the impact of vaccination
- Investigating patient experience with, and the use of, an electronic monitoring system to assess vaccination responses
- A meta-analysis of interventions that enhance the use of adult immunization and cancer screening services
- Do vaccines seem to work against bacterial and viral infections, and are they effective?
- Gathering the evidence for the introduction of typhoid vaccine: worldwide vaccine testing
- Explore molecular mimicry to broaden the immune response to carbohydrate antigens for vaccine development
- Tumor-associated glycan and immune surveillance
- Rational design and application of idiotope vaccines
- Assessing the effects of vaccines on immune-deficient people
- What are the impacts of rapid growth and deployment of high-volume vaccines for pandemic response
Anti-vaccination Research Paper Topics
- Should the state impose vaccinations, or should the choice be left up to the child’s parents?
- What is the connection between vaccination and autism?
- Is natural immunity better than immunity through immunization?
- Examining cultural perspectives on vaccination
- Are they worth it? adverse effects of vaccination on children
- To vaccinate or not against HPV? A content analysis of vocabularies of motives
- Vaccines: religious and cultural arguments from an Islamic perspective
- Anti-science populism or biomedicine’s unresolved knots? Comparing views on the movements against mandatory pediatric vaccines
- An anthropological commentary on vaccine hesitancy, decision-making, and interventionism among religious minorities
- Understanding attitudes to vaccination
Research Topics For Covid-19 Vaccination
- Medical mistrust in the context of Covid-19: implications for intended care-seeking and quarantine policy support in the United States
- What is the acceptability of the potential COVID-19 vaccine among smokers and non-smokers?
- COVID-19 vaccine hesitancy in healthcare personnel: are there any differences among classifications
- Discuss various options that one can use to convince people to get the covid-19 vaccine
- Examining COVID-19 vaccine efficacy after the first dose: Pfizer, Moderna, AstraZeneca
- Discuss the impacts of herd immunity during the covid-19 pandemic
- What are some of the effects of covid-19 vaccination on transmission of disease?
- Discuss whether antibodies generated through vaccination recognize all-new variants of covid-19
- Investigate how the intensity of lockdowns accelerate or influence mutation of the COVID virus
- Examine how the new covid-19 strain identified in England will affect the available vaccines.
- Outline which immunoglobulin types can be used as the markers for covid-19 vaccination
- Which is the best way to deal with swaps after completing vaccinations in nursing homes
- How do we curb vaccine hesitancy among healthcare providers?
- Which one is the more dangerous, covid-19 or covid-19 vaccine? What must be the individual decision?
- Analyzing Ebola and the evolving ethics of quarantine
- Break down some of the side effects of covid-19 vaccination
- How long will immunity last after receiving the covid-19 vaccination?
- Will, a covid-19 vaccine work for everyone? Are there people who cannot get vaccinated?
- Is bivalent OPV immunization capable of mitigating the impact of covid-19?
- What are the expected long-term side effects of the vaccination for covid-19?
- Evaluate differences between the first and second doses of the covid-19 mRNA vaccine?
- Examine the ingredients in the covid-19 mRNA vaccine
- Can a person’s DNA change through mRNA vaccines?
- Factors that stops the body from continuing to produce COVID-19 spike protein after getting a COVID-19 mRNA
- Discuss whether a person vaccinated against covid-19 will be able to spread the virus to susceptible people
- Investigating vaccination adverse outcomes and costs of vaccine injury claims(VICs): In the past, present, and during COVID-19.
- Who gets cured: Covid-19 and the development of critical sociology and anthropology of cure
- Development of perception and attitude scales related to COVID-19 pandemic
- Does the mutation of the coronavirus affect the capacity of the vaccines to prevent disease?
- A case-control study: finding a link between pre-existing antibodies got after the childhood vaccinations or past infections and COVID-19?
- Queue questions: ethics of COVID-19 vaccine prioritization
- Disparities between Black and White in H1N1 vaccination among adults in the U.S. in 2009: A cautionary tale for the COVID-19 pandemic
- Autonomy and refusal in pandemics: What to do with those who refuse COVID-19 vaccines
- Knowledge, attitude, and acceptance of a COVID-19 vaccine: a global cross-sectional study
- Prospects of COVID-19 vaccine implementation in the U.S.: Challenges and potential solutions
- What are the effects of COVID-19 vaccines on pregnant women?
- Compare and contrast the efficacy of different covid-19 vaccines.
- Ways to improve covid-19 vaccine acceptance
- Determination of causation between COVID-19 vaccines and potential adverse effects
Vaccination Of Children Topics
- What is the essence of increasing HPV vaccination among children?
- Analyze the primary diseases that vaccines prevent in children
- What will happen if a child’s vaccination schedule is delayed
- Look at the vaccination schedule for children in the U.S.
- Can children receive more than one vaccine at a time?
- Examine revaccination outcomes of children with proximate vaccine seizures
- What are the impacts of measles-containing vaccination in children with the severe underlying neurologic disease?
- Evaluate the challenges involved in measuring immunization activity coverage among measles zero-dose children
- What is the connection between the polio vaccine and the risk of cancer among children?
- Do multiple vaccines affect babies’ health and immune system in an adverse war, or can their bodies handle them?
- What are the various vaccination options available for children, and are they harmful to children’s overall health?
- The case for further research and development: assessing the potential cost-effectiveness of microneedle patches in childhood measles vaccination programs
- Evaluate the accuracy of parental recall of child immunization in an inner-city population
- Evaluating maternal acculturation and childhood immunization levels among children in African-American families in Florida
- Policy analysis: the impact of the vaccine for children’s program on child immunization delivery
- The effect of managed care: investigating access of infant immunizations for poor inner-city families
- Who takes up free flu shots? Investigating the effects of an expansion in coverage
- What are the societal and parental values for the risks and benefits of childhood combination vaccines?
- Looking into trends in vaccination intentions and risk perceptions: a longitudinal study of the first year of the H1N1 pandemic
Healthcare Topics About Vaccination
- Conscious consideration of herd immunity in influenza vaccination decisions
- A case study of ethnic or racial differences in Medicare experiences and immunization
- What preservatives are used in vaccines
- Discuss the relationship between vaccines and autism
- What is the role of epidemiology in infection control?
- How t design and select the most relevant immunogenic peptide sequences
- Discuss why the Zika virus has not had a significant impact in Africa as compared to America
- What are the advantages of using the phage display technology of antibodies versus hybridism technology?
- Analyzing the impact and cost-effectiveness of vaccination programs in a country using mathematical models
- Malaria vaccines: progress and problems
- Malaria: cloning genes for antigens of plasmodium falciparum
- Fighting profits on the pandemic: The fight for vaccines in today’s economic and geopolitical context
- Molecular and biotechnological approaches to fish vaccines
- Immunogenicity of a whole-cell pertussis vaccine with low lipopolysaccharide content in infants
- Immunogrid: an integrative environment for large-scale simulation of the immune system for vaccine discovery, design, and optimization
Thesis Topics In Vaccination
- Investigating challenges and opportunities in vaccine delivery, discovery, and development
- Discuss classic methods of vaccine development
- What are some of the current problems in vaccinology?
- Assess some of the latest tools for vaccine development
- Using cost-effectiveness analysis to support research and development portfolio prioritization for product innovations in measles vaccination
- Communicating vaccine safety during the introduction and development of vaccines
- Highlighting viral vectors for use in the development of biodefense vaccines
- What is the role of US. military research programs in the invention of USA-approved vaccines for naturally occurring infectious diseases
- Curbing outbreaks: utilizing international governmental risk pools to fund research and development of infectious disease medicines and vaccines
- Vaccine stabilization: research commercialization and likely impacts
- Exam the unequal interactions of the role of patient-centered care in the inequitable diffusion of medical innovation, the human papillomavirus(HPV) vaccine
- A case study of the status of development of vaccines and vaccine research for malaria
- Enteric infections vs vaccines: a public health and clinical research agenda for developing countries
- A review of research and vaccine development for industry animals in third world countries
- How the research-based industry approaches vaccine development and establishes priorities
- A look at the status of vaccine research and development of a vaccine for HIV-1
- Modeling a cost-effective vaccination strategy for the prevention of herpes zoster infection
- Using an adequate T.B. vaccination regiment to identify immune responses associated with protection in the murine model
- A systematic analysis of the link between vaccines and atopic dermatitis
- Do vaccines provide better immunity than natural infections?
- Is there a need to be vaccinated against a disease that is not available in your country or community
- How to strengthen adult immunization via coordinated action
- Using the general equilibrium method to assess the value of a malaria vaccine: An application to African countries
- Who should take up free flu shots?
- Evaluate the impact of vaccination among health care personnel
- Retail clinics and their impact on vaccination in the U.S.
- Discuss the societal values for the benefits and risks of childhood combination vaccines
- How safe and effective is the synovial vaccine for people above 60 years
- Evaluating vaccination effectiveness of group-specific fractional-dose strategies
Law Research Topics On Vaccination
- Explain why there are age restrictions for Rotavirus vaccination?
- Vaccination or hygiene : Which factor contributes to the decline of infectious diseases?
- Outline the main factors that cause vaccine failure
- Discuss why HIV is so hard to vaccinate in uninfected people?
- In what ways do maternal vaccinations affect the fetal nervous system development
- How to deliver malaria vaccine effectively and efficiently
- Highlight the vaccines that are specifically licensed in the U.S. for pregnant women
- How does an immune genetic algorithm work?
- Evaluate the relationship between the success of artificial insemination and vaccination
- Outline the reasons why vaccines underperform in low-income countries
- Discuss U.S. immigration and vaccination policy
- Assessing the effectiveness of compelled vaccination
Vaccination Ethical Topics
- What are the requirements for a strain to be used as a vaccine?
- What is the best way to administer vaccines in children?
- Assessing the benefits of maternal vaccination on breastfed infants
- Evaluating the pros and cons of intraperitoneal vaccination
- Examine ways to measure the pattern of vaccination acceptance
- Investigate Covid-19 transmission, vaccination rate, and the fate of resistant strains
- Look into dark web marketplaces and covid-19 vaccines.
- A close look at covid-19 vaccines and kidney diseases
- Contextualizing the impact of covid-19 vaccine misinformation on vaccination intent in the U.S.
- Examining behaviors and attitudes of medical students towards covid-19 vaccines
From the exceptional vaccination debate topics, titles, and other ideas above, you can quickly tell that we have your best interest at heart. We believe that all students deserve decent scores on their papers. As a result, we are more than willing to recommend affordable online help with research papers from the best-rated sites.
Whether you are overwhelmed by other assignments, dealing with a tight deadline, or lack the spark to pull it off, you can count on cheap yet professional thesis help from trusted assignment help sites. They are ready to deliver high-quality papers, and you can confirm that from any sample or example on the platforms.

Make PhD experience your own
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
- Scoping Review
- Open access
- Published: 14 November 2021
Effectiveness and safety of SARS-CoV-2 vaccine in real-world studies: a systematic review and meta-analysis
- Qiao Liu 1 na1 ,
- Chenyuan Qin 1 , 2 na1 ,
- Min Liu 1 &
- Jue Liu ORCID: orcid.org/0000-0002-1938-9365 1 , 2
Infectious Diseases of Poverty volume 10 , Article number: 132 ( 2021 ) Cite this article
56k Accesses
203 Citations
372 Altmetric
Metrics details
To date, coronavirus disease 2019 (COVID-19) becomes increasingly fierce due to the emergence of variants. Rapid herd immunity through vaccination is needed to block the mutation and prevent the emergence of variants that can completely escape the immune surveillance. We aimed to systematically evaluate the effectiveness and safety of COVID-19 vaccines in the real world and to establish a reliable evidence-based basis for the actual protective effect of the COVID-19 vaccines, especially in the ensuing waves of infections dominated by variants.
We searched PubMed, Embase and Web of Science from inception to July 22, 2021. Observational studies that examined the effectiveness and safety of SARS-CoV-2 vaccines among people vaccinated were included. Random-effects or fixed-effects models were used to estimate the pooled vaccine effectiveness (VE) and incidence rate of adverse events after vaccination, and their 95% confidence intervals ( CI ).
A total of 58 studies (32 studies for vaccine effectiveness and 26 studies for vaccine safety) were included. A single dose of vaccines was 41% (95% CI : 28–54%) effective at preventing SARS-CoV-2 infections, 52% (31–73%) for symptomatic COVID-19, 66% (50–81%) for hospitalization, 45% (42–49%) for Intensive Care Unit (ICU) admissions, and 53% (15–91%) for COVID-19-related death; and two doses were 85% (81–89%) effective at preventing SARS-CoV-2 infections, 97% (97–98%) for symptomatic COVID-19, 93% (89–96%) for hospitalization, 96% (93–98%) for ICU admissions, and 95% (92–98%) effective for COVID-19-related death, respectively. The pooled VE was 85% (80–91%) for the prevention of Alpha variant of SARS-CoV-2 infections, 75% (71–79%) for the Beta variant, 54% (35–74%) for the Gamma variant, and 74% (62–85%) for the Delta variant. The overall pooled incidence rate was 1.5% (1.4–1.6%) for adverse events, 0.4 (0.2–0.5) per 10 000 for severe adverse events, and 0.1 (0.1–0.2) per 10 000 for death after vaccination.
Conclusions
SARS-CoV-2 vaccines have reassuring safety and could effectively reduce the death, severe cases, symptomatic cases, and infections resulting from SARS-CoV-2 across the world. In the context of global pandemic and the continuous emergence of SARS-CoV-2 variants, accelerating vaccination and improving vaccination coverage is still the most important and urgent matter, and it is also the final means to end the pandemic.
Graphical Abstract
Since its outbreak, coronavirus disease 2019 (COVID-19) has spread rapidly, with a sharp rise in the accumulative number of infections worldwide. As of August 8, 2021, COVID-19 has already killed more than 4.2 million people and more than 203 million people were infected [ 1 ]. Given its alarming-spreading speed and the high cost of completely relying on non-pharmaceutical measures, we urgently need safe and effective vaccines to cover susceptible populations and restore people’s lives into the original [ 2 ].
According to global statistics, as of August 2, 2021, there are 326 candidate vaccines, 103 of which are in clinical trials, and 19 vaccines have been put into normal use, including 8 inactivated vaccines and 5 protein subunit vaccines, 2 RNA vaccines, as well as 4 non-replicating viral vector vaccines [ 3 ]. Our World in Data simultaneously reported that 27.3% of the world population has received at least one dose of a COVID-19 vaccine, and 13.8% is fully vaccinated [ 4 ].
To date, COVID-19 become increasingly fierce due to the emergence of variants [ 5 , 6 , 7 ]. Rapid herd immunity through vaccination is needed to block the mutation and prevent the emergence of variants that can completely escape the immune surveillance [ 6 , 8 ]. Several reviews systematically evaluated the effectiveness and/or safety of the three mainstream vaccines on the market (inactivated virus vaccines, RNA vaccines and viral vector vaccines) based on random clinical trials (RCT) yet [ 9 , 10 , 11 , 12 , 13 ].
In general, RNA vaccines are the most effective, followed by viral vector vaccines and inactivated virus vaccines [ 10 , 11 , 12 , 13 ]. The current safety of COVID-19 vaccines is acceptable for mass vaccination, but long-term monitoring of vaccine safety is needed, especially in older people with underlying conditions [ 9 , 10 , 11 , 12 , 13 ]. Inactivated vaccines had the lowest incidence of adverse events and the safety comparisons between mRNA vaccines and viral vectors were controversial [ 9 , 10 ].
RCTs usually conduct under a very demanding research circumstance, and tend to be highly consistent and limited in terms of population characteristics and experimental conditions. Actually, real-world studies differ significantly from RCTs in terms of study conditions and mass vaccination in real world requires taking into account factors, which are far more complex, such as widely heterogeneous populations, vaccine supply, willingness, medical accessibility, etc. Therefore, the real safety and effectiveness of vaccines turn out to be a major concern of international community. The results of a mass vaccination of CoronaVac in Chile demonstrated a protective effectiveness of 65.9% against the onset of COVID-19 after complete vaccination procedures [ 14 ], while the outcomes of phase 3 trials in Brazil and Turkey were 50.7% and 91.3%, reported on Sinovac’s website [ 14 ]. As for the Delta variant, the British claimed 88% protection after two doses of BNT162b2, compared with 67% for AZD1222 [ 15 ]. What is surprising is that the protection of BNT162b2 against infection in Israel is only 39% [ 16 ]. Several studies reported the effectiveness and safety of the COVID-19 vaccine in the real world recently, but the results remain controversial [ 17 , 18 , 19 , 20 ]. A comprehensive meta-analysis based upon the real-world studies is still in an urgent demand, especially for evaluating the effect of vaccines on variation strains. In the present study, we aimed to systematically evaluate the effectiveness and safety of the COVID-19 vaccine in the real world and to establish a reliable evidence-based basis for the actual protective effect of the COVID-19 vaccines, especially in the ensuing waves of infections dominated by variants.
Search strategy and selection criteria
Our methods were described in detail in our published protocol [PROSPERO (Prospective register of systematic reviews) registration, CRD42021267110]. We searched eligible studies published by 22 July 2021, from three databases including PubMed, Embase and Web of Science by the following search terms: (effectiveness OR safety) AND (COVID-19 OR coronavirus OR SARS-CoV-2) AND (vaccine OR vaccination). We used EndNoteX9.0 (Thomson ResearchSoft, Stanford, USA) to manage records, screen and exclude duplicates. This study was strictly performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).
We included observational studies that examined the effectiveness and safety of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccines among people vaccinated with SARS-CoV-2 vaccines. The following studies were excluded: (1) irrelevant to the subject of the meta-analysis, such as studies that did not use SARS-CoV-2 vaccination as the exposure; (2) insufficient data to calculate the rate for the prevention of COVID-19, the prevention of hospitalization, the prevention of admission to the ICU, the prevention of COVID-19-related death, or adverse events after vaccination; (3) duplicate studies or overlapping participants; (4) RCT studies, reviews, editorials, conference papers, case reports or animal experiments; and (5) studies that did not clarify the identification of COVID-19.
Studies were identified by two investigators (LQ and QCY) independently following the criteria above, while discrepancies reconciled by a third investigator (LJ).
Data extraction and quality assessment
The primary outcome was the effectiveness of SARS-CoV-2 vaccines. The following data were extracted independently by two investigators (LQ and QCY) from the selected studies: (1) basic information of the studies, including first author, publication year and study design; (2) characteristics of the study population, including sample sizes, age groups, setting or locations; (3) kinds of the SARS-CoV-2 vaccines; (4) outcomes for the effectiveness of SARS-CoV-2 vaccines: the number of laboratory-confirmed COVID-19, hospitalization for COVID-19, admission to the ICU for COVID-19, and COVID-19-related death; and (5) outcomes for the safety of SARS-CoV-2 vaccines: the number of adverse events after vaccination.
We evaluated the risk of bias using the Newcastle–Ottawa quality assessment scale for cohort studies and case–control studies [ 21 ]. and assess the methodological quality using the checklist recommended by Agency for Healthcare Research and Quality (AHRQ) [ 22 ]. Cohort studies and case–control studies were classified as having low (≥ 7 stars), moderate (5–6 stars), and high risk of bias (≤ 4 stars) with an overall quality score of 9 stars. For cross-sectional studies, we assigned each item of the AHRQ checklist a score of 1 (answered “yes”) or 0 (answered “no” or “unclear”), and summarized scores across items to generate an overall quality score that ranged from 0 to 11. Low, moderate, and high risk of bias were identified as having a score of 8–11, 4–7 and 0–3, respectively.
Two investigators (LQ and QCY) independently assessed study quality, with disagreements resolved by a third investigator (LJ).
Data synthesis and statistical analysis
We performed a meta-analysis to pool data from included studies and assess the effectiveness and safety of SARS-CoV-2 vaccines by clinical outcomes (rates of the prevention of COVID-19, the prevention of hospitalization, the prevention of admission to the ICU, the prevention of COVID-19-related death, and adverse events after vaccination). Random-effects or fixed-effects models were used to pool the rates and adjusted estimates across studies separately, based on the heterogeneity between estimates ( I 2 ). Fixed-effects models were used if I 2 ≤ 50%, which represented low to moderate heterogeneity and random-effects models were used if I 2 > 50%, representing substantial heterogeneity.
We conducted subgroup analyses to investigate the possible sources of heterogeneity by using vaccine kinds, vaccination status, sample size, and study population as grouping variables. We used the Q test to conduct subgroup comparisons and variables were considered significant between subgroups if the subgroup difference P value was less than 0.05. Publication bias was assessed by funnel plot and Egger’s regression test. We analyzed data using Stata version 16.0 (StataCorp, Texas, USA).
A total of 4844 records were searched from the three databases. 2484 duplicates were excluded. After reading titles and abstracts, we excluded 2264 reviews, RCT studies, duplicates and other studies meeting our exclude criteria. Among the 96 studies under full-text review, 41 studies were excluded (Fig. 1 ). Ultimately, with three grey literatures included, this final meta-analysis comprised 58 eligible studies, including 32 studies [ 14 , 15 , 17 , 18 , 19 , 20 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 ] for vaccine effectiveness and 26 studies [ 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 ] for vaccine safety. Characteristics of included studies are showed in Additional file 1 : Table S1, Additional file 2 : Table S2. The risk of bias of all studies we included was moderate or low.
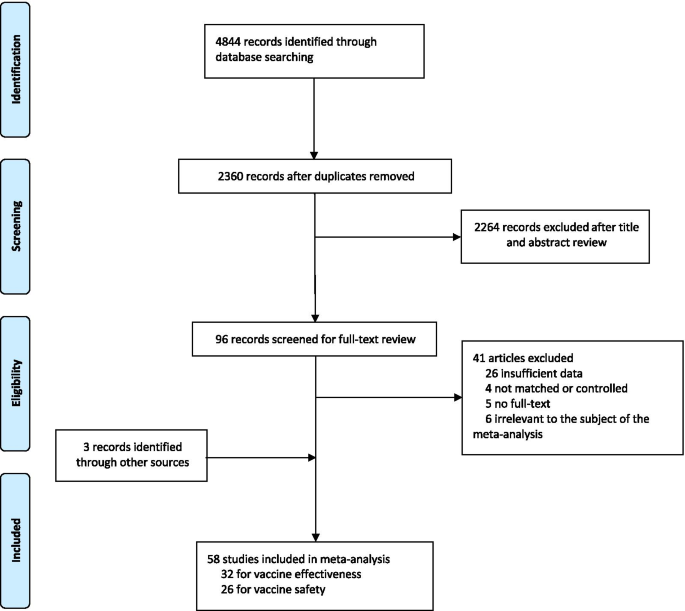
Flowchart of the study selection
Vaccine effectiveness for different clinical outcomes of COVID-19
We separately reported the vaccine effectiveness (VE) by the first and second dose of vaccines, and conducted subgroup analysis by the days after the first or second dose (< 7 days, ≥ 7 days, ≥ 14 days, and ≥ 21 days; studies with no specific days were classified as 1 dose, 2 dose or ≥ 1 dose).
For the first dose of SARS-CoV-2 vaccines, the pooled VE was 41% (95% CI : 28–54%) for the prevention of SARS-CoV-2 infection, 52% (95% CI : 31–73%) for the prevention of symptomatic COVID-19, 66% (95% CI : 50–81%) for the prevention of hospital admissions, 45% (95% CI : 42–49%) for the prevention of ICU admissions, and 53% (95% CI : 15–91%) for the prevention of COVID-19-related death (Table 1 ). The subgroup, ≥ 21 days after the first dose, was found to have the highest VE in each clinical outcome of COVID-19, regardless of ≥ 1 dose group (Table 1 ).
For the second dose of SARS-CoV-2 vaccines, the pooled VE was 85% (95% CI : 81–89%) for the prevention of SARS-CoV-2 infection, 97% (95% CI : 97–98%) for the prevention of symptomatic COVID-19, 93% (95% CI: 89–96%) for the prevention of hospital admissions, 96% (95% CI : 93–98%) for the prevention of ICU admissions, and 95% (95% CI : 92–98%) for the prevention of COVID-19-related death (Table 1 ). VE was 94% (95% CI : 78–98%) in ≥ 21 days after the second dose for the prevention of SARS-CoV-2 infection, higher than other subgroups, regardless of 2 dose group (Table 1 ). For the prevention of symptomatic COVID-19, VE was also relatively higher in 21 days after the second dose (99%, 95% CI : 94–100%). Subgroups showed no statistically significant differences in the prevention of hospital admissions, ICU admissions and COVID-19-related death (subgroup difference P values were 0.991, 0.414, and 0.851, respectively).
Vaccine effectiveness for different variants of SARS-CoV-2 in fully vaccinated people
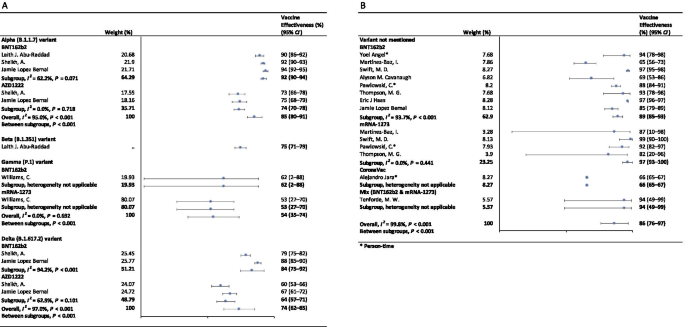
In the fully vaccinated groups (over 14 days after the second dose), the pooled VE was 85% (95% CI: 80–91%) for the prevention of Alpha variant of SARS-CoV-2 infection, 54% (95% CI : 35–74%) for the Gamma variant, and 74% (95% CI : 62–85%) for the Delta variant. There was only one study [ 23 ] focused on the Beta variant, which showed the VE was 75% (95% CI : 71–79%) for the prevention of the Beta variant of SARS-CoV-2 infection. BNT162b2 vaccine had the highest VE in each variant group; 92% (95% CI : 90–94%) for the Alpha variant, 62% (95% CI : 2–88%) for the Gamma variant, and 84% (95% CI : 75–92%) for the Delta variant (Fig. 2 ).
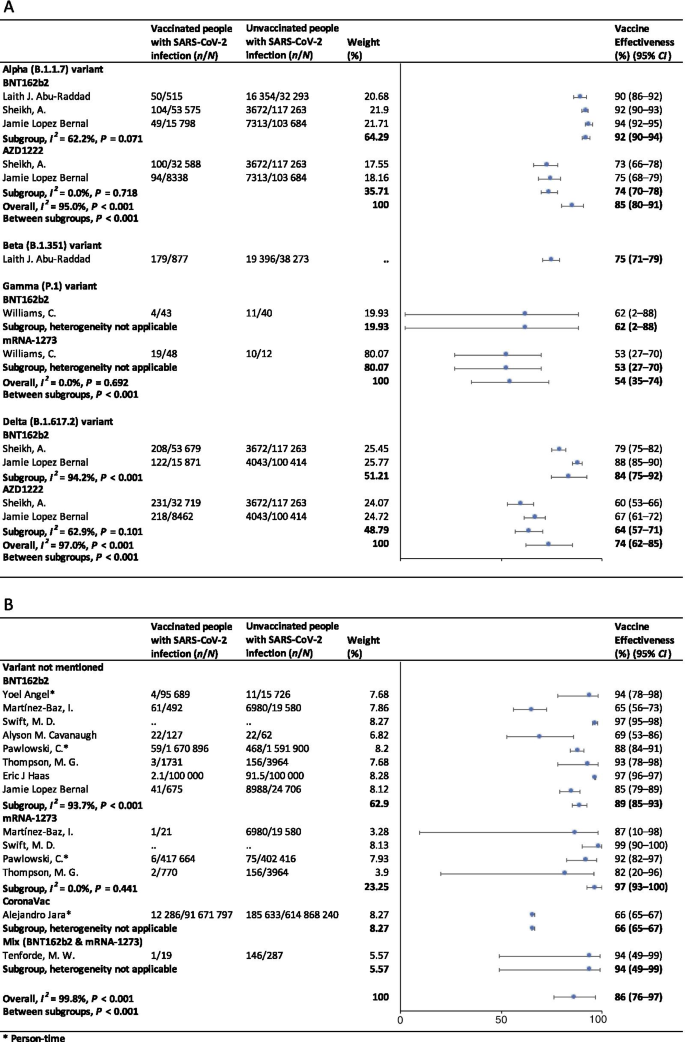
Forest plots for the vaccine effectiveness of SARS-CoV-2 vaccines in fully vaccinated populations. A Vaccine effectiveness against SARS-CoV-2 variants; B Vaccine effectiveness against SARS-CoV-2 with variants not mentioned. SARS-CoV-2 severe acute respiratory syndrome coronavirus 2, COVID-19 coronavirus disease 2019, CI confidence interval
For studies which had not mentioned the variant of SARS-CoV-2, the pooled VE was 86% (95% CI: 76–97%) for the prevention of SARS-CoV-2 infection in fully vaccinated people. mRNA-1273 vaccine had the highest pooled VE (97%, 95% CI: 93–100%, Fig. 2 ).
Safety of SARS-CoV-2 vaccines
As Table 2 showed, the incidence rate of adverse events varied widely among different studies. We conducted subgroup analysis by study population (general population, patients and healthcare workers), vaccine type (BNT162b2, mRNA-1273, CoronaVac, and et al.), and population size (< 1000, 1000–10 000, 10 000–100 000, and > 100 000). The overall pooled incidence rate was 1.5% (95% CI : 1.4–1.6%) for adverse events, 0.4 (95% CI : 0.2–0.5) per 10 000 for severe adverse events, and 0.1 (95% CI : 0.1–0.2) per 10 000 for death after vaccination. Incidence rate of adverse events was higher in healthcare workers (53.2%, 95% CI : 28.4–77.9%), AZD1222 vaccine group (79.6%, 95% CI : 60.8–98.3%), and < 1000 population size group (57.6%, 95% CI : 47.9–67.4%). Incidence rate of sever adverse events was higher in healthcare workers (127.2, 95% CI : 62.7–191.8, per 10 000), Gam-COVID-Vac vaccine group (175.7, 95% CI : 77.2–274.2, per 10 000), and 1000–10 000 population size group (336.6, 95% CI : 41.4–631.8, per 10 000). Incidence rate of death after vaccination was higher in patients (7.6, 95% CI : 0.0–32.2, per 10 000), BNT162b2 vaccine group (29.8, 95% CI : 0.0–71.2, per 10 000), and < 1000 population size group (29.8, 95% CI : 0.0–71.2, per 10 000). Subgroups of general population, vaccine type not mentioned, and > 100 000 population size had the lowest incidence rate of adverse events, severe adverse events, and death after vaccination.
Sensitivity analysis and publication bias
In the sensitivity analyses, VE for SARS-CoV-2 infections, symptomatic COVID-19 and COVID-19-related death got relatively lower when omitting over a single dose group of Maria et al.’s work [ 33 ]; when omitting ≥ 14 days after the first dose group and ≥ 14 days after the second dose group of Alejandro et al.’s work [ 14 ], VE for SARS-CoV-2 infections, hospitalization, ICU admission and COVID-19-related death got relatively higher; and VE for all clinical status of COVID-19 became lower when omitting ≥ 14 days after the second dose group of Eric et al.’s work [ 34 ]. Incidence rate of adverse events and severe adverse events got relatively higher when omitting China CDC’s data [ 74 ]. P values of Egger’s regression test for all the meta-analysis were more than 0.05, indicating that there might not be publication bias.
To our knowledge, this is a comprehensive systematic review and meta-analysis assessing the effectiveness and safety of SARS-CoV-2 vaccines based on real-world studies, reporting pooled VE for different variants of SARS-CoV-2 and incidence rate of adverse events. This meta-analysis comprised a total of 58 studies, including 32 studies for vaccine effectiveness and 26 studies for vaccine safety. We found that a single dose of SARS-CoV-2 vaccines was about 40–60% effective at preventing any clinical status of COVID-19 and that two doses were 85% or more effective. Although vaccines were not as effective against variants of SARS-CoV-2 as original virus, the vaccine effectiveness was still over 50% for fully vaccinated people. Normal adverse events were common, while the incidence of severe adverse events or even death was very low, providing reassurance to health care providers and to vaccine recipients and promote confidence in the safety of COVID-19 vaccines. Our findings strengthen and augment evidence from previous review [ 75 ], which confirmed the effectiveness of the BNT162b2 mRNA vaccine, and additionally reported the safety of SARS-CoV-2 vaccines, giving insight on the future of SARS-CoV-2 vaccine schedules.
Although most vaccines for the prevention of COVID-19 are two-dose vaccines, we found that the pooled VE of a single dose of SARS-CoV-2 vaccines was about 50%. Recent study showed that the T cell and antibody responses induced by a single dose of the BNT162b2 vaccine were comparable to those naturally infected with SARE-CoV-2 within weeks or months after infection [ 76 ]. Our findings could help to develop vaccination strategies under certain circumstances such as countries having a shortage of vaccines. In some countries, in order to administer the first dose to a larger population, the second dose was delayed for up to 12 weeks [ 77 ]. Some countries such as Canada had even decided to delay the second dose for 16 weeks [ 78 ]. However, due to a suboptimum immune response in those receiving only a single dose of a vaccine, such an approach had a chance to give rise to the emergence of variants of SARS-CoV-2 [ 79 ]. There remains a need for large clinical trials to assess the efficacy of a single-dose administration of two-dose vaccines and the risk of increasing the emergence of variants.
Two doses of SARS-CoV-2 vaccines were highly effective at preventing hospitalization, severe cases and deaths resulting from COVID-19, while the VE of different groups of days from the second vaccine dose showed no statistically significant differences. Our findings emphasized the importance of getting fully vaccinated, for the fact that most breakthrough infections were mild or asymptomatic. A recent study showed that the occurrence of breakthrough infections with SARS-CoV-2 in fully vaccinated populations was predictable with neutralizing antibody titers during the peri-infection period [ 80 ]. We also found getting fully vaccinated was at least 50% effective at preventing SARS-CoV-2 variants infections, despite reduced effectiveness compared with original virus; and BNT162b2 vaccine was found to have the highest VE in each variant group. Studies showed that the highly mutated variants were indicative of a form of rapid, multistage evolutionary jumps, which could preferentially occur in the milieu of partial immune control [ 81 , 82 ]. Therefore, immunocompromised patients should be prioritized for anti-COVID-19 immunization to mitigate persistent SARS-CoV-2 infections, during which multimutational SARS-CoV-2 variants could arise [ 83 ].
Recently, many countries, including Israel, the United States, China and the United Kingdom, have introduced a booster of COVID-19 vaccine, namely the third dose [ 84 , 85 , 86 , 87 ]. A study of Israel showed that among people vaccinated with BNT162b2 vaccine over 60 years, the risk of COVID-19 infection and severe illness in the non-booster group was 11.3 times (95% CI: 10.4–12.3) and 19.5 times (95% CI: 12.9–29.5) than the booster group, respectively [ 84 ]. Some studies have found that the third dose of Moderna, Pfizer-BioNTech, Oxford-AstraZeneca and Sinovac produced a spike in infection-blocking neutralizing antibodies when given a few months after the second dose [ 85 , 87 , 88 ]. In addition, the common adverse events associated with the third dose did not differ significantly from the symptoms of the first two doses, ranging from mild to moderate [ 85 ]. The overall incidence rate of local and systemic adverse events was 69% (57/97) and 20% (19/97) after receiving the third dose of BNT162b2 vaccine, respectively [ 88 ]. Results of a phase 3 clinical trial involving 306 people aged 18–55 years showed that adverse events after receiving a third dose of BNT162b2 vaccine (5–8 months after completion of two doses) were similar to those reported after receiving a second dose [ 85 ]. Based on V-safe, local reactions were more frequently after dose 3 (5323/6283; 84.7%) than dose 2 (5249/6283; 83.5%) among people who received 3 doses of Moderna. Systemic reactions were reported less frequently after dose 3 (4963/6283; 79.0%) than dose 2 (5105/6283; 81.3%) [ 86 ]. On August 4, WHO called for a halt to booster shots until at least the end of September to achieve an even distribution of the vaccine [ 89 ]. At this stage, the most important thing we should be thinking about is how to reach a global cover of people at risk with the first or second dose, rather than focusing on the third dose.
Based on real world studies, our results preliminarily showed that complete inoculation of COVID-19 vaccines was still effective against infection of variants, although the VE was generally diminished compared with the original virus. Particularly, the pooled VE was 54% (95% CI : 35–74%) for the Gamma variant, and 74% (95% CI : 62–85%) for the Delta variant. Since the wide spread of COVID-19, a number of variants have drawn extensive attention of international community, including Alpha variant (B.1.1.7), first identified in the United Kingdom; Beta variant (B.1.351) in South Africa; Gamma variant (P.1), initially appeared in Brazil; and the most infectious one to date, Delta variant (B.1.617.2) [ 90 ]. Israel recently reported a breakthrough infection of SARS-CoV-2, dominated by variant B.1.1.7 in a small number of fully vaccinated health care workers, raising concerns about the effectiveness of the original vaccine against those variants [ 80 ]. According to an observational cohort study in Qatar, VE of the BNT162b2 vaccine against the Alpha (B.1.1.7) and Beta (B.1.351) variants was 87% (95% CI : 81.8–90.7%) and 75.0% (95% CI : 70.5–7.9%), respectively [ 23 ]. Based on the National Immunization Management System of England, results from a recent real-world study of all the general population showed that the AZD1222 and BNT162b2 vaccines protected against symptomatic SARS-CoV-2 infection of Alpha variant with 74.5% (95% CI : 68.4–79.4%) and 93.7% (95% CI : 91.6–95.3%) [ 15 ]. In contrast, the VE against the Delta variant was 67.0% (95% CI : 61.3–71.8%) for two doses of AZD1222 vaccine and 88% (95% CI : 85.3–90.1%) for BNT162b2 vaccine [ 15 ].
In terms of adverse events after vaccination, the pooled incidence rate was very low, only 1.5% (95% CI : 1.4–1.6%). However, the prevalence of adverse events reported in large population (population size > 100 000) was much lower than that in small to medium population size. On the one hand, the vaccination population in the small to medium scale studies we included were mostly composed by health care workers, patients with specific diseases or the elderly. And these people are more concerned about their health and more sensitive to changes of themselves. But it remains to be proved whether patients or the elderly are more likely to have adverse events than the general. Mainstream vaccines currently on the market have maintained robust safety in specific populations such as cancer patients, organ transplant recipients, patients with rheumatic and musculoskeletal diseases, pregnant women and the elderly [ 54 , 91 , 92 , 93 , 94 ]. A prospective study by Tal Goshen-lag suggests that the safety of BNT162b2 vaccine in cancer patients is consistent with those previous reports [ 91 ]. In addition, the incidence rate of adverse events reported in the heart–lung transplant population is even lower than that in general population [ 95 ]. On the other hand, large scale studies at the national level are mostly based on national electronic health records or adverse event reporting systems, and it is likely that most mild or moderate symptoms are actually not reported.
Compared with the usual local adverse events (such as pain at the injection site, redness at the injection site, etc.) and normal systemic reactions (such as fatigue, myalgia, etc.), serious and life-threatening adverse events were rare due to our results. A meta-analysis based on RCTs only showed three cases of anaphylactic shock among 58 889 COVID-19 vaccine recipients and one in the placebo group [ 11 ]. The exact mechanisms underlying most of the adverse events are still unclear, accordingly we cannot establish a causal relation between severe adverse events and vaccination directly based on observational studies. In general, varying degrees of adverse events occur after different types of COVID-19 vaccination. Nevertheless, the benefits far outweigh the risks.
Our results showed the effectiveness and safety of different types of vaccines varied greatly. Regardless of SARS-CoV-2 variants, vaccine effectiveness varied from 66% (CoronaVac [ 14 ]) to 97% (mRNA-1273 [ 18 , 20 , 45 , 46 ]). The incidence rate of adverse events varied widely among different types of vaccines, which, however, could be explained by the sample size and population group of participants. BNT162b2, AZD1222, mRNA-1273 and CoronaVac were all found to have high vaccine efficacy and acceptable adverse-event profile in recent published studies [ 96 , 97 , 98 , 99 ]. A meta-analysis, focusing on the potential vaccine candidate which have reached to the phase 3 of clinical development, also found that although many of the vaccines caused more adverse events than the controls, most were mild, transient and manageable [ 100 ]. However, severe adverse events did occur, and there remains the need to implement a unified global surveillance system to monitor the adverse events of COVID-19 vaccines around the world [ 101 ]. A recent study employed a knowledge-based or rational strategy to perform a prioritization matrix of approved COVID-19 vaccines, and led to a scale with JANSSEN (Ad26.COV2.S) in the first place, and AZD1222, BNT162b2, and Sputnik V in second place, followed by BBIBP-CorV, CoronaVac and mRNA-1273 in third place [ 101 ]. Moreover, when deciding the priority of vaccines, the socioeconomic characteristics of each country should also be considered.
Our meta-analysis still has several limitations. First, we may include limited basic data on specific populations, as vaccination is slowly being promoted in populations under the age of 18 or over 60. Second, due to the limitation of the original real-world study, we did not conduct subgroup analysis based on more population characteristics, such as age. When analyzing the efficacy and safety of COVID-19 vaccine, we may have neglected the discussion on the heterogeneity from these sources. Third, most of the original studies only collected adverse events within 7 days after vaccination, which may limit the duration of follow-up for safety analysis.
Based on the real-world studies, SARS-CoV-2 vaccines have reassuring safety and could effectively reduce the death, severe cases, symptomatic cases, and infections resulting from SARS-CoV-2 across the world. In the context of global pandemic and the continuous emergence of SARS-CoV-2 variants, accelerating vaccination and improving vaccination coverage is still the most important and urgent matter, and it is also the final means to end the pandemic.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its additional information files.
Abbreviations
Coronavirus disease 2019
Severe Acute Respiratory Syndrome Coronavirus 2
Vaccine effectiveness
Confidence intervals
Intensive care unit
Random clinical trials
Preferred reporting items for systematic reviews and meta-analyses
COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). 2021. https://coronavirus.jhu.edu/map.html . Accessed 20 Aug 2021.
Barranco R, Rocca G, Molinelli A, Ventura F. Controversies and challenges of mass vaccination against SARS-CoV-2 in Italy: medico-legal perspectives and considerations. Healthcare (Basel). 2021. https://doi.org/10.3390/healthcare9091163 .
Article Google Scholar
COVID-19 vaccine tracker. 2021. https://vac-lshtm.shinyapps.io/ncov_vaccine_landscape/ . Accessed 20 Aug 2021.
Coronavirus (COVID-19) Vaccinations. 2021. https://ourworldindata.org/covid-vaccinations . Accessed 20 Aug 2021.
Kirby T. New variant of SARS-CoV-2 in UK causes surge of COVID-19. Lancet Respir Med. 2021;9(2):e20–1. https://doi.org/10.1016/s2213-2600(21)00005-9 .
Article CAS PubMed PubMed Central Google Scholar
Callaway E. Fast-spreading COVID variant can elude immune responses. Nature. 2021;589(7843):500–1. https://doi.org/10.1038/d41586-021-00121-z .
Article CAS PubMed Google Scholar
Reardon S. How the Delta variant achieves its ultrafast spread. Nature. 2021. https://doi.org/10.1038/d41586-021-01986-w .
Article PubMed Google Scholar
Li R, Liu J, Zhang H. The challenge of emerging SARS-CoV-2 mutants to vaccine development. J Genet Genomics. 2021;48(2):102–6. https://doi.org/10.1016/j.jgg.2021.03.001 .
Article PubMed PubMed Central Google Scholar
Chen M, Yuan Y, Zhou Y, Deng Z, Zhao J, Feng F, Zou H, Sun C. Safety of SARS-CoV-2 vaccines: a systematic review and meta-analysis of randomized controlled trials. Infect Dis Poverty. 2021;10(1):94. https://doi.org/10.1186/s40249-021-00878-5 .
Ling Y, Zhong J, Luo J. Safety and effectiveness of SARS-CoV-2 vaccines: a systematic review and meta-analysis. J Med Virol. 2021. https://doi.org/10.1002/jmv.27203 .
Pormohammad A, Zarei M, Ghorbani S, Mohammadi M, Razizadeh MH, Turner DL, Turner RJ. Efficacy and safety of COVID-19 vaccines: a systematic review and meta-analysis of randomized clinical trials. Vaccines (Basel). 2021. https://doi.org/10.3390/vaccines9050467 .
Sathian B, Asim M, Banerjee I, Roy B, Pizarro AB, Mancha MA, van Teijlingen ER, Kord-Varkaneh H, Mekkodathil AA, Subramanya SH, et al. Development and implementation of a potential coronavirus disease 2019 (COVID-19) vaccine: a systematic review and meta-analysis of vaccine clinical trials. Nepal J Epidemiol. 2021;11(1):959–82. https://doi.org/10.3126/nje.v11i1.36163 .
Yuan P, Ai P, Liu Y, Ai Z, Wang Y, Cao W, Xia X, Zheng JC. Safety, tolerability, and immunogenicity of COVID-19 vaccines: a systematic review and meta-analysis. medRxiv. 2020. https://doi.org/10.1101/2020.11.03.20224998 .
Jara A, Undurraga EA, González C, Paredes F, Fontecilla T, Jara G, Pizarro A, Acevedo J, Leo K, Leon F, et al. Effectiveness of an inactivated SARS-CoV-2 vaccine in Chile. N Engl J Med. 2021. https://doi.org/10.1056/NEJMoa2107715 .
Lopez Bernal J, Andrews N, Gower C, Gallagher E, Simmons R, Thelwall S, Stowe J, Tessier E, Groves N, Dabrera G, et al. Effectiveness of COVID-19 vaccines against the B.1.617.2 (Delta) variant. N Engl J Med. 2021. https://doi.org/10.1056/NEJMoa2108891 .
Israel says Pfizer Covid vaccine is just 39% effective as delta spreads, but still prevents severe illness. 2021. https://www.cnbc.com/2021/07/23/delta-variant-pfizer-covid-vaccine-39percent-effective-in-israel-prevents-severe-illness.html . Accessed 20 Aug 2021.
Zacay G, Shasha D, Bareket R, Kadim I, Hershkowitz Sikron F, Tsamir J, Mossinson D, Heymann AD. BNT162b2 vaccine effectiveness in preventing asymptomatic infection with SARS-CoV-2 virus: a nationwide historical cohort study. Open Forum Infect Dis. 2021;8(6): ofab262. https://doi.org/10.1093/ofid/ofab262 .
Martínez-Baz I, Miqueleiz A, Casado I, Navascués A, Trobajo-Sanmartín C, Burgui C, Guevara M, Ezpeleta C, Castilla J. Effectiveness of COVID-19 vaccines in preventing SARS-CoV-2 infection and hospitalisation, Navarre, Spain, January to April 2021. Eurosurveillance. 2021. https://doi.org/10.2807/1560-7917.Es.2021.26.21.2100438 .
Tenforde MW, Olson SM, Self WH, Talbot HK, Lindsell CJ, Steingrub JS, Shapiro NI, Ginde AA, Douin DJ, Prekker ME, et al. Effectiveness of Pfizer-BioNTech and moderna vaccines against COVID-19 among hospitalized adults aged ≥65 years—United States, January–March 2021. MMWR Morb Mortal Wkly Rep. 2021;70(18):674–9. https://doi.org/10.15585/mmwr.mm7018e1 .
Pawlowski C, Lenehan P, Puranik A, Agarwal V, Venkatakrishnan AJ, Niesen MJM, O’Horo JC, Virk A, Swift MD, Badley AD, et al. FDA-authorized mRNA COVID-19 vaccines are effective per real-world evidence synthesized across a multi-state health system. Med (N Y). 2021. https://doi.org/10.1016/j.medj.2021.06.007 .
Wells G, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp . Accessed 20 Aug 2021.
Rostom A, Dubé C, Cranney A, et al. Celiac Disease. Rockville (MD): Agency for Healthcare Research and Quality (US); 2004 Sep. (Evidence Reports/Technology Assessments, No. 104.) Appendix D. Quality Assessment Forms. Available from: https://www.ncbi.nlm.nih.gov/books/NBK35156/ . Accessed 20 Aug 2021
Abu-Raddad LJ, Chemaitelly H, Butt AA. Effectiveness of the BNT162b2 COVID-19 vaccine against the B.1.1.7 and B.1.351 Variants. N Engl J Med. 2021;385(2):187–9. https://doi.org/10.1056/NEJMc2104974 .
Angel Y, Spitzer A, Henig O, Saiag E, Sprecher E, Padova H, Ben-Ami R. Association between vaccination with BNT162b2 and incidence of symptomatic and asymptomatic SARS-CoV-2 infections among health care workers. JAMA. 2021;325(24):2457–65. https://doi.org/10.1001/jama.2021.7152 .
Azamgarhi T, Hodgkinson M, Shah A, Skinner JA, Hauptmannova I, Briggs TWR, Warren S. BNT162b2 vaccine uptake and effectiveness in UK healthcare workers—a single centre cohort study. Nat Commun. 2021;12(1):3698. https://doi.org/10.1038/s41467-021-23927-x .
Bianchi FP, Germinario CA, Migliore G, Vimercati L, Martinelli A, Lobifaro A, Tafuri S, Stefanizzi P. BNT162b2 mRNA COVID-19 vaccine effectiveness in the prevention of SARS-CoV-2 infection: a preliminary report. J Infect Dis. 2021. https://doi.org/10.1093/infdis/jiab262 .
Britton A, Jacobs Slifka KM, Edens C, Nanduri SA, Bart SM, Shang N, Harizaj A, Armstrong J, Xu K, Ehrlich HY, et al. Effectiveness of the Pfizer-BioNTech COVID-19 vaccine among residents of two skilled nursing facilities experiencing COVID-19 outbreaks—Connecticut, December 2020–February 2021. MMWR Morb Mortal Wkly Rep. 2021;70(11):396–401. https://doi.org/10.15585/mmwr.mm7011e3 .
Cavanaugh AM, Fortier S, Lewis P, Arora V, Johnson M, George K, Tobias J, Lunn S, Miller T, Thoroughman D, et al. COVID-19 outbreak associated with a SARS-CoV-2 R1 lineage variant in a skilled nursing facility after vaccination program—Kentucky, March 2021. MMWR Morb Mortal Wkly Rep. 2021;70(17):639–43. https://doi.org/10.15585/mmwr.mm7017e2 .
Chemaitelly H, Yassine HM, Benslimane FM, Al Khatib HA, Tang P, Hasan MR, Malek JA, Coyle P, Ayoub HH, Al Kanaani Z, et al. mRNA-1273 COVID-19 vaccine effectiveness against the B.1.1.7 and B.1.351 variants and severe COVID-19 disease in Qatar. Nat Med. 2021. https://doi.org/10.1038/s41591-021-01446-y .
Chodick G, Tene L, Patalon T, Gazit S, Ben Tov A, Cohen D, Muhsen K. Assessment of effectiveness of 1 dose of BNT162b2 vaccine for SARS-CoV-2 infection 13 to 24 days after immunization. JAMA Netw Open. 2021;4(6): e2115985. https://doi.org/10.1001/jamanetworkopen.2021.15985 .
Chodick G, Tene L, Rotem RS, Patalon T, Gazit S, Ben-Tov A, Weil C, Goldshtein I, Twig G, Cohen D, et al. The effectiveness of the TWO-DOSE BNT162b2 vaccine: analysis of real-world data. Clin Infect Dis. 2021. https://doi.org/10.1093/cid/ciab438 .
Dagan N, Barda N, Kepten E, Miron O, Perchik S, Katz MA, Hernán MA, Lipsitch M, Reis B, Balicer RD. BNT162b2 mRNA COVID-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384(15):1412–23. https://doi.org/10.1056/NEJMoa2101765 .
Flacco ME, Soldato G, Acuti Martellucci C, Carota R, Di Luzio R, Caponetti A, Manzoli L. Interim estimates of COVID-19 vaccine effectiveness in a mass vaccination setting: data from an Italian Province. VacCInes (Basel). 2021. https://doi.org/10.3390/vaccines9060628 .
Haas EJ, Angulo FJ, McLaughlin JM, Anis E, Singer SR, Khan F, Brooks N, Smaja M, Mircus G, Pan K, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397(10287):1819–29. https://doi.org/10.1016/s0140-6736(21)00947-8 .
Hall VJ, Foulkes S, Saei A, Andrews N, Oguti B, Charlett A, Wellington E, Stowe J, Gillson N, Atti A, et al. COVID-19 vaccine coverage in health-care workers in England and effectiveness of BNT162b2 mRNA vaccine against infection (SIREN): a prospective, multicentre, cohort study. Lancet. 2021;397(10286):1725–35. https://doi.org/10.1016/s0140-6736(21)00790-x .
Hyams C, Marlow R, Maseko Z, King J, Ward L, Fox K, Heath R, Tuner A, Friedrich Z, Morrison L, et al. Effectiveness of BNT162b2 and ChAdOx1 nCoV-19 COVID-19 vaccination at preventing hospitalisations in people aged at least 80 years: a test-negative, case-control study. Lancet Infect Dis. 2021. https://doi.org/10.1016/s1473-3099(21)00330-3 .
Khan N, Mahmud N. Effectiveness of SARS-CoV-2 vaccination in a veterans affairs cohort of patients with inflammatory bowel disease with diverse exposure to immunosuppressive medications. Gastroenterology. 2021. https://doi.org/10.1053/j.gastro.2021.05.044 .
Knobel P, Serra C, Grau S, Ibañez R, Diaz P, Ferrández O, Villar R, Lopez AF, Pujolar N, Horcajada JP, et al. COVID-19 mRNA vaccine effectiveness in asymptomatic healthcare workers. Infect Control Hosp Epidemiol. 2021. https://doi.org/10.1017/ice.2021.287 .
Lopez Bernal J, Andrews N, Gower C, Robertson C, Stowe J, Tessier E, Simmons R, Cottrell S, Roberts R, O’Doherty M, et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. BMJ. 2021;373: n1088. https://doi.org/10.1136/bmj.n1088 .
Mazagatos C, Monge S, Olmedo C, Vega L, Gallego P, Martín-Merino E, Sierra MJ, Limia A, Larrauri A. Effectiveness of mRNA COVID-19 vaccines in preventing SARS-CoV-2 infections and COVID-19 hospitalisations and deaths in elderly long-term care facility residents, Spain, weeks 53, 2020 to 13 2021. Eurosurveillance. 2021. https://doi.org/10.2807/1560-7917.Es.2021.26.24.2100452 .
Pilishvili T, Fleming-Dutra KE, Farrar JL, Gierke R, Mohr NM, Talan DA, Krishnadasan A, Harland KK, Smithline HA, Hou PC, et al. Interim estimates of vaccine effectiveness of Pfizer-BioNTech and Moderna COVID-19 vaccines among health care personnel—33 US Sites, January–March 2021. MMWR Morb Mortal Wkly Rep. 2021;70(20):753–8. https://doi.org/10.15585/mmwr.mm7020e2 .
Sheikh A, McMenamin J, Taylor B, Robertson C. SARS-CoV-2 Delta VOC in Scotland: demographics, risk of hospital admission, and vaccine effectiveness. Lancet. 2021;397(10293):2461–2. https://doi.org/10.1016/s0140-6736(21)01358-1 .
Shrotri M, Krutikov M, Palmer T, Giddings R, Azmi B, Subbarao S, Fuller C, Irwin-Singer A, Davies D, Tut G, et al. Vaccine effectiveness of the first dose of ChAdOx1 nCoV-19 and BNT162b2 against SARS-CoV-2 infection in residents of long-term care facilities in England (VIVALDI): a prospective cohort study. Lancet Infect Dis. 2021. https://doi.org/10.1016/s1473-3099(21)00289-9 .
Skowronski DM, Setayeshgar S, Zou M, Prystajecky N, Tyson JR, Galanis E, Naus M, Patrick DM, Sbihi H, El Adam S, et al. Single-dose mRNA vaccine effectiveness against SARS-CoV-2, including Alpha and Gamma variants: a test-negative design in adults 70 years and older in British Columbia,Canada. Clin Infect Dis. 2021. https://doi.org/10.1093/cid/ciab616 .
Swift MD, Breeher LE, Tande AJ, Tommaso CP, Hainy CM, Chu H, Murad MH, Berbari EF, Virk A. Effectiveness of mRNA COVID-19 vaccines against SARS-CoV-2 infection in a cohort of healthcare personnel. Clin Infect Dis. 2021. https://doi.org/10.1093/cid/ciab361 .
Thompson MG, Burgess JL, Naleway AL, Tyner H, Yoon SK, Meece J, Olsho LEW, Caban-Martinez AJ, Fowlkes AL, Lutrick K, et al. Prevention and attenuation of COVID-19 with the BNT162b2 and mRNA-1273 Vaccines. N Engl J Med. 2021. https://doi.org/10.1056/NEJMoa2107058 .
Vasileiou E, Simpson CR, Shi T, Kerr S, Agrawal U, Akbari A, Bedston S, Beggs J, Bradley D, Chuter A, et al. Interim findings from first-dose mass COVID-19 vaccination roll-out and COVID-19 hospital admissions in Scotland: a national prospective cohort study. Lancet. 2021;397(10285):1646–57. https://doi.org/10.1016/s0140-6736(21)00677-2 .
Williams C, Al-Bargash D, Macalintal C, Stuart R, Seth A, Latham J, Gitterman L, Fedsin S, Godoy M, Kozak R, et al. COVID-19 outbreak associated with a SARS-CoV-2 P.1 lineage in a long-term care home after implementation of a vaccination program—Ontario, April–May 2021. Clin Infect Dis. 2021. https://doi.org/10.1093/cid/ciab617 .
Alhazmi A, Alamer E, Daws D, Hakami M, Darraj M, Abdelwahab S, Maghfuri A, Algaissi A. Evaluation of side effects associated with COVID-19 vaccines in Saudi Arabia. Vaccines (Basel). 2021. https://doi.org/10.3390/vaccines9060674 .
Andrzejczak-Grządko S, Czudy Z, Donderska M. Side effects after COVID-19 vaccinations among residents of Poland. Eur Rev Med Pharmacol Sci. 2021;25(12):4418–21. https://doi.org/10.26355/eurrev_202106_26153 .
Baldolli A, Michon J, Appia F, Galimard C, Verdon R, Parienti JJ. Tolerance of BNT162b2 mRNA COVI-19 vaccine in patients with a medical history of COVID-19 disease: a case control study. Vaccine. 2021;39(32):4410–3. https://doi.org/10.1016/j.vaccine.2021.06.054 .
Cherian S, Paul A, Ahmed S, Alias B, Manoj M, Santhosh AK, Varghese DR, Krishnan N, Shenoy P. Safety of the ChAdOx1 nCoV-19 and the BBV152 vaccines in 724 patients with rheumatic diseases: a post-vaccination cross-sectional survey. Rheumatol Int. 2021;41(8):1441–5. https://doi.org/10.1007/s00296-021-04917-0 .
Chevallier P, Coste-Burel M, Le Bourgeois A, Peterlin P, Garnier A, Béné MC, Imbert BM, Drumel T, Le Gouill S, Moreau P, et al. Safety and immunogenicity of a first dose of SARS-CoV-2 mRNA vaccine in allogeneic hematopoietic stem-cells recipients. EJHaem. 2021. https://doi.org/10.1002/jha2.242 .
Connolly CM, Ruddy JA, Boyarsky BJ, Avery RK, Werbel WA, Segev DL, Garonzik-Wang J, Paik JJ. Safety of the first dose of mRNA SARS-CoV-2 vaccines in patients with rheumatic and musculoskeletal diseases. Ann Rheum Dis. 2021. https://doi.org/10.1136/annrheumdis-2021-220231 .
Furer V, Eviatar T, Zisman D, Peleg H, Paran D, Levartovsky D, Zisapel M, Elalouf O, Kaufman I, Meidan R, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis. 2021. https://doi.org/10.1136/annrheumdis-2021-220647 .
Gee J, Marquez P, Su J, Calvert GM, Liu R, Myers T, Nair N, Martin S, Clark T, Markowitz L, et al. First month of COVID-19 vaccine safety monitoring—United States, December 14, 2020–January 13, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(8):283–8. https://doi.org/10.15585/mmwr.mm7008e3 .
Hashimoto T, Ozaki A, Bhandari D, Sawano T, Sah R, Tanimoto T. High anaphylaxis rates following vaccination with the Pfizer BNT162b2 mRNA vaccine against COVID-19 in Japanese health care workers; a secondary analysis of initial post-approval safety data. J Travel Med. 2021. https://doi.org/10.1093/jtm/taab090 .
Lv G, Yuan J, Xiong X, Li M. Mortality rate and characteristics of deaths following COVID-19 vaccination. Front Med (Lausanne). 2021;8: 670370. https://doi.org/10.3389/fmed.2021.670370 .
McMurry R, Lenehan P, Awasthi S, Silvert E, Puranik A, Pawlowski C, Venkatakrishnan AJ, Anand P, Agarwal V, O’Horo JC, et al. Real-time analysis of a mass vaccination effort confirms the safety of FDA-authorized mRNA COVID-19 vaccines. Med (N Y). 2021. https://doi.org/10.1016/j.medj.2021.06.006 .
Monin L, Laing AG, Muñoz-Ruiz M, McKenzie DR, Del Molino Del Barrio I, Alaguthurai T, Domingo-Vila C, Hayday TS, Graham C, Seow J, et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: interim analysis of a prospective observational study. Lancet Oncol. 2021;22(6):765–78. https://doi.org/10.1016/s1470-2045(21)00213-8 .
Pagotto V, Ferloni A, Mercedes Soriano M, Díaz M, Braguinsky Golde N, González MI, Asprea V, Staneloni MI, Zingoni P, Vidal G, et al. Active monitoring of early safety of Sputnik V vaccine in Buenos Aires, Argentina. MediCIna (B Aires). 2021;81(3):408–14.
Google Scholar
Peled Y, Ram E, Lavee J, Sternik L, Segev A, Wieder-Finesod A, Mandelboim M, Indenbaum V, Levy I, Raanani E, et al. BNT162b2 vaccination in heart transplant recipients: Clinical experience and antibody response. J Heart Lung Transplant. 2021. https://doi.org/10.1016/j.healun.2021.04.003 .
Quiroga B, Sánchez-Álvarez E, Goicoechea M, de Sequera P. COVID-19 vaccination among Spanish nephrologists: acceptance and side effects. J Healthc Qual Res. 2021. https://doi.org/10.1016/j.jhqr.2021.05.002 .
Ram R, Hagin D, Kikozashvilli N, Freund T, Amit O, Bar-On Y, Beyar-Katz O, Shefer G, Moshiashvili MM, Karni C, et al. Safety and immunogenicity of the BNT162b2 mRNA COVID-19 vaccine in patients after allogeneic HCT or CD19-based CART therapy—a single center prospective cohort study. Transplant Cell Ther. 2021. https://doi.org/10.1016/j.jtct.2021.06.024 .
Revon-Riviere G, Ninove L, Min V, Rome A, Coze C, Verschuur A, de Lamballerie X, André N. The BNT162b2 mRNA COVID-19 vaccine in adolescents and young adults with cancer: a monocentric experience. Eur J Cancer. 2021;154:30–4. https://doi.org/10.1016/j.ejca.2021.06.002 .
Riad A, Pokorná A, Mekhemar M, Conrad J, Klugarová J, Koščík M, Klugar M, Attia S. Safety of ChAdOx1 nCoV-19 vaccine: independent evidence from two EU states. Vaccines (Basel). 2021. https://doi.org/10.3390/vaccines9060673 .
Riad A, Sağıroğlu D, Üstün B, Pokorná A, Klugarová J, Attia S, Klugar M. Prevalence and risk factors of CoronaVac Side effects: an independent cross-sectional study among healthcare workers in Turkey. J Clin Med. 2021. https://doi.org/10.3390/jcm10122629 .
Rosman Y, Lavi N, Meir-Shafrir K, Lachover-Roth I, Cohen-Engler A, Mekori YA, Confino-Cohen R. Safety of BNT162b2 mRNA COVID-19 vaccine in patients with mast cell disorders. J Allergy Clin Immunol Pract. 2021. https://doi.org/10.1016/j.jaip.2021.06.032 .
Signorelli C, Odone A, Gianfredi V, Capraro M, Kacerik E, Chiecca G, Scardoni A, Minerva M, Mantecca R, Musarò P, et al. Application of the “immunization islands” model to improve quality, efficiency and safety of a COVID-19 mass vaccination site. Ann Ig. 2021;33(5):499–512. https://doi.org/10.7416/ai.2021.2456 .
Vallée A, Chan-Hew-Wai A, Bonan B, Lesprit P, Parquin F, Catherinot É, Choucair J, Billard D, Amiel-Taieb C, Camps È, et al. Oxford-AstraZeneca COVID-19 vaccine: need of a reasoned and effective vaccine campaign. Public Health. 2021;196:135–7. https://doi.org/10.1016/j.puhe.2021.05.030 .
Wang J, Hou Z, Liu J, Gu Y, Wu Y, Chen Z, Ji J, Diao S, Qiu Y, Zou S, et al. Safety and immunogenicity of COVID-19 vaccination in patients with non-alcoholic fatty liver disease (CHESS2101): a multicenter study. J Hepatol. 2021. https://doi.org/10.1016/j.jhep.2021.04.026 .
Zhang MX, Zhang TT, Shi GF, Cheng FM, Zheng YM, Tung TH, Chen HX. Safety of an inactivated SARS-CoV-2 vaccine among healthcare workers in China. Expert Rev Vaccines. 2021. https://doi.org/10.1080/14760584.2021.1925112 .
Shay DK, Gee J, Su JR, Myers TR, Marquez P, Liu R, Zhang B, Licata C, Clark TA, Shimabukuro TT. Safety monitoring of the Janssen (Johnson & Johnson) COVID-19 Vaccine—United States, March–April 2021. MMWR Morb Mortal Wkly Rep. 2021;70(18):680–4. https://doi.org/10.15585/mmwr.mm7018e2 .
Prevention CCfDCa. Information analysis of COVID-19 vaccine adverse reaction monitoring in China. 2021-5-28. http://www.chinacdc.cn/jkzt/ymyjz/ymyjjz_6758/202105/t20210528_230908.html . Accessed 20 Aug 2021.
Kow CS, Hasan SS. Real-world effectiveness of BNT162b2 mRNA vaccine: a meta-analysis of large observational studies. Inflammopharmacology. 2021;29(4):1075–90. https://doi.org/10.1007/s10787-021-00839-2 .
Angyal A, Longet S, Moore S, Payne RP, Harding A et al. T-Cell and Antibody Responses to First BNT162b2 Vaccine Dose in Previously SARS-CoV-2-Infected and Infection-Naive UK Healthcare Workers: A Multicentre, Prospective, Observational Cohort Study. Available at SSRN: https://ssrn.com/abstract=3820576 or https://doi.org/10.2139/ssrn.3820576 . Accessed 20 Aug 2021.
Pimenta D, Yates C, Pagel C, Gurdasani D. Delaying the second dose of covid-19 vaccines. BMJ. 2021;372: n710. https://doi.org/10.1136/bmj.n710 .
Tauh T, Mozel M, Meyler P, Lee SM. An updated look at the 16-week window between doses of vaccines in BC for COVID-19. BC Med J. 2021;63(3):102–3.
Kadire SR, Wachter RM, Lurie N. Delayed second dose versus standard regimen for COVID-19 vaccination. N Engl J Med. 2021;384(9): e28. https://doi.org/10.1056/NEJMclde2101987 .
Bergwerk M, Gonen T, Lustig Y, Amit S, Lipsitch M, Cohen C, Mandelboim M, Gal Levin E, Rubin C, Indenbaum V, et al. COVID-19 breakthrough infections in vaccinated health care workers. N Engl J Med. 2021. https://doi.org/10.1056/NEJMoa2109072 .
Truong TT, Ryutov A, Pandey U, Yee R, Goldberg L, Bhojwani D, Aguayo-Hiraldo P, Pinsky BA, Pekosz A, Shen L, et al. Persistent SARS-CoV-2 infection and increasing viral variants in children and young adults with impaired humoral immunity. medRxiv. 2021. https://doi.org/10.1101/2021.02.27.21252099 .
Choi B, Choudhary MC, Regan J, Sparks JA, Padera RF, Qiu X, Solomon IH, Kuo HH, Boucau J, Bowman K, et al. Persistence and evolution of SARS-CoV-2 in an Immunocompromised Host. N Engl J Med. 2020;383(23):2291–3. https://doi.org/10.1056/NEJMc2031364 .
Corey L, Beyrer C, Cohen MS, Michael NL, Bedford T, Rolland M. SARS-CoV-2 variants in patients with immunosuppression. N Engl J Med. 2021;385(6):562–6. https://doi.org/10.1056/NEJMsb2104756 .
Bar-On YM, Goldberg Y, Mandel M, Bodenheimer O, Freedman L, Kalkstein N, Mizrahi B, Alroy-Preis S, Ash N, Milo R, et al. Protection of BNT162b2 vaccine booster against Covid-19 in Israel. N Engl J Med. 2021;385(15):1393–400. https://doi.org/10.1056/NEJMoa2114255 .
Hause AM, Baggs J, Gee J, Marquez P, Myers TR, Shimabukuro TT, Shay DK. Safety monitoring of an additional dose of COVID-19 vaccine—United States, August 12–September 19, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(39):1379–84. https://doi.org/10.15585/mmwr.mm7039e4 .
Furlow B. Immunocompromised patients in the USA and UK should receive third dose of COVID-19 vaccine. Lancet Rheumatol. 2021. https://doi.org/10.1016/s2665-9913(21)00313-1 .
Flaxman A, Marchevsky NG, Jenkin D, Aboagye J, Aley PK, Angus B, Belij-Rammerstorfer S, Bibi S, Bittaye M, Cappuccini F, et al. Reactogenicity and immunogenicity after a late second dose or a third dose of ChAdOx1 nCoV-19 in the UK: a substudy of two randomised controlled trials (COV001 and COV002). Lancet. 2021;398(10304):981–90. https://doi.org/10.1016/s0140-6736(21)01699-8 .
Peled Y, Ram E, Lavee J, Segev A, Matezki S, Wieder-Finesod A, Halperin R, Mandelboim M, Indenbaum V, Levy I, et al. Third dose of the BNT162b2 vaccine in heart transplant recipients: immunogenicity and clinical experience. J Heart Lung Transplant. 2021. https://doi.org/10.1016/j.healun.2021.08.010 .
WHO. WHO press conference on coronavirus disease (COVID-19)—4 August 2021. 2021. https://www.who.int/multi-media/details/who-press-conference-on-coronavirus-disease-(covid-19)---4-august-2021 . Accessed 20 Aug 2021.
Cascella M, Rajnik M, Aleem A, Dulebohn SC, Di Napoli R. Features, evaluation, and treatment of coronavirus (COVID-19). In: StatPearls. edn. Treasure Island (FL): StatPearls Publishing Copyright © 2021, StatPearls Publishing LLC.; 2021.
Goshen-Lago T, Waldhorn I, Holland R, Szwarcwort-Cohen M, Reiner-Benaim A, Shachor-Meyouhas Y, Hussein K, Fahoum L, Baruch M, Peer A, et al. Serologic status and toxic effects of the SARS-CoV-2 BNT162b2 vaccine in patients undergoing treatment for cancer. JAMA Oncol. 2021. https://doi.org/10.1001/jamaoncol.2021.2675 .
Ou MT, Boyarsky BJ, Motter JD, Greenberg RS, Teles AT, Ruddy JA, Krach MR, Jain VS, Werbel WA, Avery RK, et al. Safety and reactogenicity of 2 doses of SARS-CoV-2 vaccination in solid organ transplant recipients. Transplantation. 2021. https://doi.org/10.1097/tp.0000000000003780 .
Bookstein Peretz S, Regev N, Novick L, Nachshol M, Goffer E, Ben-David A, Asraf K, Doolman R, Sapir E, Regev Yochay G, et al. Short-term outcome of pregnant women vaccinated by BNT162b2 mRNA COVID-19 vaccine. Ultrasound Obstet Gynecol. 2021. https://doi.org/10.1002/uog.23729 .
Shimabukuro TT, Kim SY, Myers TR, Moro PL, Oduyebo T, Panagiotakopoulos L, Marquez PL, Olson CK, Liu R, Chang KT, et al. Preliminary findings of mRNA COVID-19 vaccine safety in pregnant persons. N Engl J Med. 2021;384(24):2273–82. https://doi.org/10.1056/NEJMoa2104983 .
Peled Y, Ram E, Lavee J, Sternik L, Segev A, Wieder-Finesod A, Mandelboim M, Indenbaum V, Levy I, Raanani E, et al. BNT162b2 vaccination in heart transplant recipients: clinical experience and antibody response. J Heart Lung Transplant. 2021;40(8):759–62. https://doi.org/10.1016/j.healun.2021.04.003 .
Thomas SJ, Moreira ED Jr, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Polack FP, Zerbini C, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine through 6 months. N Engl J Med. 2021. https://doi.org/10.1056/NEJMoa2110345 .
Falsey AR, Sobieszczyk ME, Hirsch I, Sproule S, Robb ML, Corey L, Neuzil KM, Hahn W, Hunt J, Mulligan MJ, et al. Phase 3 safety and efficacy of AZD1222 (ChAdOx1 nCoV-19) COVID-19 vaccine. N Engl J Med. 2021. https://doi.org/10.1056/NEJMoa2105290 .
El Sahly HM, Baden LR, Essink B, Doblecki-Lewis S, Martin JM, Anderson EJ, Campbell TB, Clark J, Jackson LA, Fichtenbaum CJ, et al. Efficacy of the mRNA-1273 SARS-CoV-2 vaccine at completion of blinded phase. N Engl J Med. 2021. https://doi.org/10.1056/NEJMoa2113017 .
Tanriover MD, Doğanay HL, Akova M, Güner HR, Azap A, Akhan S, Köse Ş, Erdinç F, Akalın EH, Tabak ÖF, et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet. 2021;398(10296):213–22. https://doi.org/10.1016/s0140-6736(21)01429-x .
Kumar S, Saurabh MK, Maharshi V. Efficacy and safety of potential vaccine candidates against coronavirus disease 2019: a systematic review. J Adv Pharm Technol Res. 2021;12(3):215–21. https://doi.org/10.4103/japtr.JAPTR_229_20 .
Burgos-Salcedo J. A rational strategy to support approved COVID-19 vaccines prioritization. Hum Vaccin Immunother. 2021;17(10):3474–7. https://doi.org/10.1080/21645515.2021.1922060 .
Download references
Acknowledgements
This study was funded by the National Natural Science Foundation of China (72122001; 71934002) and the National Science and Technology Key Projects on Prevention and Treatment of Major infectious disease of China (2020ZX10001002). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the paper. No payment was received by any of the co-authors for the preparation of this article.
Author information
Qiao Liu and Chenyuan Qin are joint first authors
Authors and Affiliations
Department of Epidemiology and Biostatistics, School of Public Health, Peking University, Beijing, 100191, China
Qiao Liu, Chenyuan Qin, Min Liu & Jue Liu
Institute for Global Health and Development, Peking University, Beijing, 100871, China
Chenyuan Qin & Jue Liu
You can also search for this author in PubMed Google Scholar
Contributions
LQ and QCY contributed equally as first authors. LJ and LM contributed equally as correspondence authors. LJ and LM conceived and designed the study; LQ, QCY and LJ carried out the literature searches, extracted the data, and assessed the study quality; LQ and QCY performed the statistical analysis and wrote the manuscript; LJ, LM, LQ and QCY revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Correspondence to Min Liu or Jue Liu .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Supplementary Information
Additional file 1: table s1..
Characteristic of studies included for vaccine effectiveness.
Additional file 2: Table S2.
Characteristic of studies included for vaccine safety.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Liu, Q., Qin, C., Liu, M. et al. Effectiveness and safety of SARS-CoV-2 vaccine in real-world studies: a systematic review and meta-analysis. Infect Dis Poverty 10 , 132 (2021). https://doi.org/10.1186/s40249-021-00915-3
Download citation
Received : 07 September 2021
Accepted : 01 November 2021
Published : 14 November 2021
DOI : https://doi.org/10.1186/s40249-021-00915-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Effectiveness
- Meta-analysis
Infectious Diseases of Poverty
ISSN: 2049-9957
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
Loading metrics
Open Access
Peer-reviewed
Research Article
COVID-19 and vaccine hesitancy: A longitudinal study
Roles Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing
* E-mail: [email protected]
Affiliation Rady School of Management, University of California San Diego, La Jolla, California, United States of America
Roles Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing
Roles Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Visualization, Writing – original draft, Writing – review & editing
- Ariel Fridman,
- Rachel Gershon,
- Ayelet Gneezy

- Published: April 16, 2021
- https://doi.org/10.1371/journal.pone.0250123
- Peer Review
- Reader Comments
How do attitudes toward vaccination change over the course of a public health crisis? We report results from a longitudinal survey of United States residents during six months (March 16 –August 16, 2020) of the COVID-19 pandemic. Contrary to past research suggesting that the increased salience of a disease threat should improve attitudes toward vaccines, we observed a decrease in intentions of getting a COVID-19 vaccine when one becomes available. We further found a decline in general vaccine attitudes and intentions of getting the influenza vaccine. Analyses of heterogeneity indicated that this decline is driven by participants who identify as Republicans, who showed a negative trend in vaccine attitudes and intentions, whereas Democrats remained largely stable. Consistent with research on risk perception and behavior, those with less favorable attitudes toward a COVID-19 vaccination also perceived the virus to be less threatening. We provide suggestive evidence that differential exposure to media channels and social networks could explain the observed asymmetric polarization between self-identified Democrats and Republicans.
Citation: Fridman A, Gershon R, Gneezy A (2021) COVID-19 and vaccine hesitancy: A longitudinal study. PLoS ONE 16(4): e0250123. https://doi.org/10.1371/journal.pone.0250123
Editor: Valerio Capraro, Middlesex University, UNITED KINGDOM
Received: November 12, 2020; Accepted: February 14, 2021; Published: April 16, 2021
Copyright: © 2021 Fridman et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: All data and code are publicly available on the Open Science Framework at https://osf.io/kgvdy/ .
Funding: UC San Diego Global Health Initiative (GHI): awarded to all authors; Project number: 1001288. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. https://medschool.ucsd.edu/som/medicine/divisions/idgph/research/Global-Health/grant-recipients/2019-2020/Pages/Faculty-Postdoc-Travel-and-Research.aspx .
Competing interests: The authors have declared that no competing interests exist.
Introduction
Vaccinations are among the most important public health tools for reducing the spread and harm caused by dangerous diseases [ 1 ]. The World Health Organization estimates that vaccines prevented at least 10 million deaths between 2010–2015 worldwide [ 2 ]. Despite considerable evidence showing vaccines are safe [ 3 , 4 ], there is increasing skepticism toward vaccination [ 5 , 6 ]. Vaccine hesitancy has led to a decline in vaccine uptake and to an increase in the prevalence of vaccine-preventable diseases (VPDs) [ 7 , 8 ]. Ironically, the objection to vaccines is commonly a consequence of their effectiveness—because individuals have lower exposure to VPDs, they are less concerned about contracting them [ 9 ], which consequently leads to greater vaccine hesitancy [ 10 ]. The COVID-19 pandemic has created a new reality where individuals are faced with a previously unknown disease and its effects, providing a unique opportunity to investigate vaccine attitudes during a period of heightened disease salience. The present research reports findings from a longitudinal study conducted during the COVID-19 health crisis, in which we measured changes in attitudes toward a prospective vaccine, as well as shifts in vaccine attitudes in general.
Factors influencing vaccine attitudes and behaviors
Past research has identified a variety of situational and individual-level factors that influence vaccine attitudes and behavior, the most prominent of which are risk perceptions and demographic characteristics.
Assessments of risk are influenced by both cognitive evaluations (i.e., objective features of the situation such as probabilities of outcomes) and affective reactions [ 11 ], as well as by contextual factors (e.g., the information that is most available or salient at the time [ 12 ]). For example, research shows that media coverage plays a significant role in determining the extent to which we take threats seriously [ 13 ]. When individuals perceive heightened risk of a threat, they become more favorable toward interventions that mitigate that threat, including vaccination (for a meta-analysis on the effect of perceived risk on intentions and behaviors, see [ 14 ]). In the case of COVID-19, this would suggest more positive attitudes toward a vaccine and greater likelihood to get vaccinated. Indeed, research suggests that individuals should exhibit a greater interest in vaccinations during a pandemic because disease threat is more salient [ 15 ].
Past efforts to improve vaccine attitudes have had limited success or even backfired; for example, messages refuting claims about the link between vaccines and autism, as well as messages featuring images of children who were sick with VPDs, had negative effects on vaccine attitudes among those who were already hesitant to vaccinate [ 16 ]. In contrast, messaging that increases disease threat salience has shown promise in reducing vaccine hesitancy [ 5 ], and there is evidence suggesting that increased threat salience for a particular disease may also increase intentions to vaccinate for other diseases [ 17 ]. Building on these findings, we expected to find an increase in pro-vaccine attitudes and in individuals’ interest in a COVID-19 vaccine when the perceived threat of the COVID-19 virus increased.
Vaccine attitudes are also influenced by a variety of demographic and ideological factors (for a review, see [ 18 ]). For example, perceptions of vaccine risk differ among individuals of different ethnic backgrounds [ 19 ], and there is extant work demonstrating a positive correlation between socioeconomic status (SES) and vaccine hesitancy [ 20 , 21 ]. Socio-demographic factors are also linked to vaccine-related behaviors: among college students, those whose parents have attained a higher level of education are more likely to get immunized [ 22 ], and researchers have identified age as a predictor for receiving the influenza vaccine [ 23 ].
Political ideology is another well-documented determinant of vaccine-related attitudes and behaviors. Despite a common belief that liberals tend toward anti-vaccination attitudes in the United States, there is strong evidence that this trend is more present among conservatives [ 24 , 25 ]. According to a recent Gallup Poll, Republicans are twice as likely to believe the widely debunked myth that vaccines cause autism [ 26 ]. Recent work has shown that exposure to anti-vaccination tweets by President Trump—the first known U.S. president to publicly express anti-vaccination attitudes—has led to increased concern about vaccines among his supporters [ 27 ]. Based on these findings, and in conjunction with the sentiments expressed by the White House that diminished the significance of the pandemic [ 28 ], we expected to find diverging trends between Democrats and Republicans.
The current research
We collected vaccine-related attitudes of individuals living in the U.S. over a six-month period. Beginning in March 2020, we elicited attitudes from a cohort of the same individuals every month. We began data collection before any COVID-19 lockdown measures were in place (i.e., prior to the nation’s first shelter-in-place order [ 29 ]). Hence, our data spans the early phase of the pandemic, when there were fewer than 2,000 total confirmed cases in the U.S., through the following six months, at which point cumulative cases reached over 5.3 million [ 30 ].
Despite our prediction—that a public health crisis would increase disease threat, consequently increasing pro-vaccine attitudes and interest in vaccination—our data show an overall decrease in favorable attitudes toward vaccines. A closer look at the data revealed that political orientation explains more variance than any other socio-demographic variable. Specifically, participants who identify as Republican showed a decrease in their intention to get the COVID-19 vaccine and the influenza vaccine as well as a general decrease in pro-vaccine attitudes, whereas Democrats’ responses to these measures did not show a significant change during this period.
Our work is the first, to our knowledge, to longitudinally measure individuals’ attitudes toward vaccines. In doing so, our findings advance the understanding of how vaccine attitudes might change during an unprecedented public health crisis, revealing a strong association between political party affiliation and vaccine attitudes.
Participants
We recruited a panel of U.S. residents on Amazon’s Mechanical Turk platform to respond to multiple survey waves. To incentivize completion of all waves, we informed participants their payment would increase for subsequent surveys. Participants were paid 30 cents for wave 1, 40 cents for wave 2, and 60 cents for waves 3 and 4, $1.00 for wave 5, and $1.20 for wave 6. In addition, participants were informed that those who completed the first three waves would enter a $100 raffle. The median survey completion time was 5.5 minutes. The sample size for the first wave was 1,018, and the number of participants ranged from 608–762 on subsequent waves (see S1 Table for attrition details). This project was certified as exempt from IRB review by the University of California, San Diego Human Research Protections Program (Project #191273XX).
Our panel represents the broad and diverse population of the United States. The first wave sample included participants from all 50 states (except Wyoming) and Washington D.C., with an age range of 18 to 82 years old (mean = 38.48, median = 35). Approximately half (53%) identified as male, 46% as female, and.6% as other. The racial makeup in our sample was: 80% White, 9% Asian, 6% Black or African American, 4% multiple racial or ethnic identities, and 1% other. Relative to the U.S. Census (2019) [ 31 ] estimates, our sample over-represents White and Asian individuals, and under-represents Black or African American individuals and other racial groups.
We elicited political affiliation using a 6-point Likert scale, ranging from Strongly Republican to Strongly Democratic. In wave 1, 62% identified as Democrats and 38% identified as Republican, which is consistent with results from the most recent General Social Survey (GSS) [ 32 ]. There was no significant change in mean political identity from wave 1 to waves 2–6 (see S2 Table ). We classified participants as Democrats or Republicans using wave 1 political party affiliation. See S2 Appendix for additional details about the correlation of political party affiliation with age, gender, and SES.
Questions and measures
Our primary measure of interest was participants’ stated intention to get the COVID-19 vaccine when it becomes available. We were also interested in their perceptions of COVID-19 threat, general vaccination attitudes, and intention to get the flu shot. For all measures, except flu shot intentions, we combined multiple items to create a composite measure (see S2 Table for specific questions and construct compositions). Questions designed to measure general vaccination attitudes were adapted from prior work [ 33 ].
Additional measures of interest were participants’ trust in broad institutions (media, local government, and federal government). These trust measures followed different trends from each other, and therefore were not combined. At the end of the survey, participants responded to demographic questions. We retained all questions used in wave 1 throughout all six waves (our survey included additional items not reported in this paper; see S2 and S3 Tables for a complete list of measured items).
Data and analysis plan
Only participants with non-missing and non-duplicated responses were included in the analyses (see S1 Appendix for additional details). For all outcomes of interest, we tested for linear trends over time using a fixed effects regression specification [ 34 ]. All regression results include individual-level fixed effects, and standard errors are clustered at the individual level, to adjust for within-person correlation. We used this approach to control for the impact of omitted or unobserved time-invariant variables. P-values are not adjusted for multiple testing (see [ 35 ]). All analyses were conducted using R (version 4.0.2), and regressions were run using the package “fixest” (version 0.6.0). All materials, data, and additional analyses including robustness checks can be found here: https://osf.io/kgvdy/ .
We report results for three different vaccination-related measures: attitudes toward a COVID-19 vaccine, general vaccination attitudes, and flu shot intentions. All measures showed a decreasing trend (Ps < .001, except flu shot intentions where p = .05) for the 6-month duration of the study, indicating a reduction in pro-vaccination attitudes and intention to get vaccinated (COVID-19 and influenza vaccines). See S4 Table for full results of all regressions.
Heterogeneity in trend by political party
To better understand whether the decline in vaccine attitudes over time was driven by a particular factor, we used a data-driven approach, regressing all demographic characteristics on vaccine attitudes, in separate regressions. These demographics included education, income, SES, race, gender, an item measuring whether participants considered themselves to be a minority, whether the participant has children, and political party. Education, income, and SES were median split; race and gender were dummy coded; and political party affiliation was dichotomized into Democrat or Republican. Among all demographic characteristics, separating time trends by political affiliation (by adding an interaction term) attained the greatest adjusted within-R 2 in explaining vaccination attitude measures. In other words, political party affiliation explains the greatest within-individual variation in vaccine attitudes over time.
An analysis of responses by political affiliation revealed that the observed decreasing trend in all three vaccine measures was mostly driven by participants who identified as Republican (all Ps < .05), whereas Democrats’ responses showed either no significant trend (for COVID-19 vaccination and flu shot intentions: Ps >.67) or a significantly less negative time trend (general vaccination: p < .001). For these regressions, and moving forward, all results included interactions between wave and political party as well as interactions for wave and age, and wave and SES, to control for potentially different time trends associated with these variables. In each regression we also tested whether the strength of political affiliation moderates the observed results, and we reported the result when it did. We also conducted ANOVAs to compare mean responses for the outcomes of interest between Democrats and Republicans, separately for each wave (see S5 Table ).
COVID-19 vaccination attitudes ( Fig 1 , Panel A).
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
Points represent means, and error bars represent 95% confidence intervals. All scale responses range from 1 to 7.
https://doi.org/10.1371/journal.pone.0250123.g001
A two-item construct ( r = .78) was created, with greater values corresponding to more favorable responses.
In wave 1, Democrats ( M = 5.39, SD = 1.55) had more favorable attitudes toward a COVID-19 vaccine than Republicans ( M = 4.57, SD = 1.76; t = -7.38, p < .001, d = -.48, 95% CI = [-.61, -.35]). Among Democrats, there was no significant time trend ( β = .02, SE = .04, p >.67) whereas Republicans’ responses followed a decreasing time trend ( β = -.09, SE = .05, p = .046). These trends were significantly different from each other ( β = -.11, SE = .02, p < .001).
General vaccination attitudes ( Fig 1 , Panel B).
A ten-item construct ( α = .95) was created, with greater values corresponding to a more positive attitude toward vaccination in general.
In wave 1, Democrats ( M = 5.83, SD = 1.15) expressed more favorable general vaccination attitudes than Republicans ( M = 5.17, SD = 1.31; t = -7.91, p < .001, d = -.52, 95% CI = [-.66, -.39]). Although both Democrats and Republicans had a decreasing time trend (Democrats: β = -.04, SE = .02, p = .029; Republicans: β = -.09, SE = .02, p < .001), the trend for Republicans was significantly more negative ( β = -.04, SE = .01, p < .001).
Flu shot intentions ( Fig 1 , Panel C).
We asked participants whether they plan to get the flu shot next year, with greater values indicating greater intentions.
In wave 1, Democrats ( M = 4.84, SD = 2.34) indicated greater intentions to vaccinate against the flu than Republicans ( M = 4.35, SD = 2.39; t = -3.15, p = .002, d = -.21, 95% CI = [-.34, -.08]). Among Democrats, there was no significant time trend ( β = .01, SE = .04, p = .86), suggesting their vaccination intentions remained largely stable. Republicans’ responses, however, revealed a decreasing time trend ( β = -.12, SE = .04, p = .005), and the two trends were significantly different from each other ( β = -.12, SE = .02, p < .001).
Our analyses revealed an interaction with political affiliation strength among Republicans, whereby participants who identified as more strongly Republican had a more negative time trend ( β = -.05, SE = .02, p = .027). This interaction was not significant for Democrats ( β = -.02, SE = .01, p = .19).
Perceived threat of COVID-19 ( Fig 2 ).
https://doi.org/10.1371/journal.pone.0250123.g002
A three-item construct ( α = .82) was created, with greater perceived threat about COVID-19.
In wave 1, Democrats ( M = 4.26, SD = 1.25) expressed greater perceived threat of COVID-19 than Republicans ( M = 3.90, SD = 1.39; t = -4.14, p < .001, d = -.40, 95% CI = [-.27, -.14]). Democrats’ responses showed an increasing time trend ( β = .08, SE = .04, p = .033), indicating they became increasingly concerned about the threat posed by the virus over time. Among Republicans, there was no significant time trend ( β = -.01, SE = .04, p = .83). These trends were significantly different from each other ( β = -.09, SE = .02, p < .001). While our data does not render causal claims, it is possible that the divergence in COVID-19 threat perceptions over time among Republicans and Democrats contributes to the divergence in vaccine attitudes between these groups over time. We revisit this proposition in the General Discussion.
Our analyses revealed an interaction with political affiliation strength among Democrats—participants who identified as more strongly Democrat had a more positive time trend ( β = .03, SE = .01, p = .019), suggesting an increasing threat perception over time. This interaction was not significant for Republicans ( β = .01, SE = .02, p = .61).
Trust in broad institutions.
The measures of trust in media, local government, and federal government were not highly correlated ( α = .66), and were therefore analyzed separately.
Trust in media ( Fig 3 , Panel A) . In wave 1, Democrats ( M = 3.61, SD = 1.66) reported greater trust in the media than Republicans ( M = 2.73, SD = 1.65; t = -8.12, p < .001, d = -.53, 95% CI = [-.66, -.39]). There was no significant time trend for either Democrats ( β = .02, SE = .04, p = .57) or Republicans ( β = -.05, SE = .04, p = .20). However, the trend for Republicans was significantly more negative ( β = -.07, SE = .02, p < .001). The different trends we observe for Democrats and Republicans with respect to trust in the media may explain the divergence in perceived threat and vaccine attitudes between these groups over time (see General discussion ).
https://doi.org/10.1371/journal.pone.0250123.g003
Trust in local government ( Fig 3 , Panel B) . In wave 1, Democrats ( M = 4.07, SD = 1.60) indicated lower trust in local government than Republicans ( M = 4.28, SD = 1.60; t = 2.01, p = .045, d = .13, 95% CI = [.003,.26]). Among Democrats, there was no significant time trend ( β = -.06, SE = .04, p = .18), though among Republicans, there was a decreasing time trend ( β = -.11, SE = .05, p = .015). These trends were significantly different from each other ( β = -.06, SE = .02, p = .004).
Trust in federal government ( Fig 3 , Panel C) . In wave 1, Democrats ( M = 2.96, SD = 1.67) expressed lower trust in the federal government than Republicans ( M = 4.08, SD = 1.60; t = 10.52, p < .001, d = .68, 95% CI = [.55,.82]). Both Democrats and Republicans had decreasing time trends (Democrats: β = -.08, SE = .04, p = .036; Republicans: β = -.10, SE = .04, p = .025). These trends were not significantly different from each other ( β = -.02, SE = .02, p = .37).
To rule out differential attrition, we tested whether the composition of our sample (i.e., age, gender, and political party) changed over time (see S1 Table ). Specifically, we tested whether participants who responded to waves 2–6 were significantly different at baseline (wave 1) from the full sample at baseline. The only significant change detected (Ps < .05) was with respect to participants’ age, though the differences were small—the average age was 38.5 at baseline, and remained between 39.9 and 40.8 at baseline among participants who responded to subsequent waves. We found no other systematic pattern of attrition among our participants.
General discussion
Over the course of six months of the COVID-19 pandemic, beginning with a relatively early phase prior to any U.S. directives to stay home (March 2020) and continuing through a cumulation of over 5 million cases (August 2020), we found a decrease in pro-vaccine attitudes and COVID-19 vaccination intentions, as well as reduced intentions to get the influenza vaccine. These findings are contrary to our prediction that increased salience of COVID-19 would improve attitudes toward vaccines.
Our analyses identify political ideology as the best predictor of the decreasing time trend across our three vaccine-related attitudes and intentions measures. In particular, we found that while Democrats’ responses remained fairly stable over time, Republicans shifted away from their lower initial responses and from Democrats’ responses, leading to increased polarization throughout the six-month period.
Contrary to the polarization observed in our data, social and behavioral scientists have long argued that groups facing threats often come together, demonstrating stronger social cohesion [ 36 ], and more cooperative behaviors [ 37 , 38 ]. Researchers have also found that individuals’ sense of shared identity plays a role in promoting cooperative behavior in response to threat [ 39 – 41 ]. Considering our results in the context of these findings might suggest that our respondents’ sense of shared identity was dominated by their political ideology, as opposed to a broader (e.g. American) identity.
What might be going on?
Although the nature of our data does not render causal claims, it highlights potential explanations. First, we note that participants’ ratings of perceived COVID-19 threat followed a similar diverging pattern by party affiliation to our three vaccine-related measures during the study period. Democrats perceived COVID-19 threat to be greater at the start of the study than Republicans did, and this gap widened significantly as the study progressed. This trend is consistent with previous research showing that vaccine hesitancy is related to perceived risk of a threat; when a VPD threat level is low, individuals are less motivated to take preventative action (i.e., immunize; for a review, see [ 42 ]).
Our data offers one potential explanation for the polarization of threat perception: Republican and Democratic participants in our study reported consuming different sources of information. The most commonly checked news source for Republicans was Fox News (Republicans: 50%, Democrats: 8%; χ 2 = 164.55, p < .001) and for Democrats was CNN (Democrats: 47%, Republicans: 23%, χ 2 = 43.08, p < .001, see S6 Table ). Corroborating this proposition, a Pew Research Center poll conducted in March 2020 found that 56% of respondents whose main news source is Fox News believed that “the news media have greatly exaggerated the risks about the Coronavirus outbreak,” whereas this was only true for 25% of those whose main news source is CNN [ 43 ]. Of note, Facebook and Instagram, were also in the top four most consumed news sources for participants affiliated with either party. Extant work describes these platforms as echo chambers [ 44 , 45 ], which may exacerbate partisan exposure to news and information.
Another trend highlighted by our data shows that similar to vaccine attitudes, Republicans’ trust in the media decreased significantly more during our study than Democrats’, suggesting these patterns might be related. According to Dr. Heidi Larson, an expert on vaccine hesitancy and founder of the Vaccine Confidence Project, misinformation regarding vaccinations is more likely to take root when individuals do not trust the information source [ 46 ]. Future research might further examine the role of trust in the media on vaccine attitudes.
While trust in media or media exposure may be driving COVID-19 threat perceptions and vaccine attitudes, there are many other possible explanatory factors that are not captured by our data or analyses. For example, it is possible that threat perceptions were influenced by how a respondents’ county or state was affected by COVID-19; up until June 2020, COVID-19 cases were more common in Democrat-leaning states [ 47 ], which might have amplified its salience early on and influenced attitudes and behavior. Further, although we included individual-level fixed-effects which control for all time invariant participant characteristics, and controlled for different trends by age and SES, we cannot rule out the possibility that other factors (e.g., educational attainment or population density) may have influenced the observed trends. Finally, as our data collection began after the onset of COVID-19, it is possible that the trend we observe for Republicans represents a return to a pre-pandemic baseline of vaccine-related attitudes.
Contributions
This work advances our understanding of how health-related attitudes evolve over time. Our focus on vaccine-related attitudes and intentions is important because experts agree that having enough people vaccinate against COVID-19 is key to stemming the pandemic [ 48 ]. More broadly, negative attitudes toward vaccination in general, and reduced vaccine uptake, is increasingly a public health concern [ 49 ]. This research provides insight into the trends of such vaccine hesitancy, underlining the importance of risk salience and its roots in ideology and media exposure.
This work also contributes to our understanding of political parties and polarization. Numerous anecdotes and reports have demonstrated a partisan divide in Americans’ response to the COVID-19 pandemic. For example, research found greater negative affective responses to wearing a face covering among politically right (vs. left) leaning individuals [ 50 ]. Here, we show that although these observations are valid, the reality is more nuanced. For example, our analyses reveal that polarization on vaccine measures—both attitudes and intentions—is driven primarily by self-identified Republicans’ gradual movement away from their initial responses whereas Democrats’ responses remained largely stable. This insight has important practical implications: It informs us about the dynamics of individuals’ attitudes, bringing us closer to understanding the underlying factors that influence attitudes and behaviors. Equipped with this knowledge, one could design more effective communications and interventions.
Note on methodology and data availability
The present study contributes to a small but growing literature in the social sciences using longitudinal data [ 51 ]. Using a longitudinal methodology allowed us to track individual-level changes over time. Merely observing a single point in time would allow us to observe across-group differences, but would lack the bigger picture of how polarization between these groups evolved. Another key advantage of panel data is that it allows us to include individual-level fixed effects, which control for the impact of omitted or unobserved time-invariant variables. Finally, panel data allows for more accurate inference of model parameters [ 52 ].
While the focus of this paper is vaccine attitudes, our broad dataset offers a unique opportunity to understand attitudes and behavior over time. Due to the richness of our data, its unique nature, and its timeliness, we believe it is important to make it available to other researchers interested in exploring it and publishing additional findings. The complete dataset is available at https://osf.io/kgvdy/ (see S2 and S3 Tables for all items collected).
Supporting information
S1 appendix. additional information about sample exclusions..
https://doi.org/10.1371/journal.pone.0250123.s001
S2 Appendix. Additional information about political party affiliation.
https://doi.org/10.1371/journal.pone.0250123.s002
S1 Table. Attrition table.
https://doi.org/10.1371/journal.pone.0250123.s003
S2 Table. Summary table of measures and constructs included in the text.
https://doi.org/10.1371/journal.pone.0250123.s004
S3 Table. Summary table of measures excluded from the text.
https://doi.org/10.1371/journal.pone.0250123.s005
S4 Table. Regression results.
https://doi.org/10.1371/journal.pone.0250123.s006
S5 Table. Outcome measures by political party affiliation.
https://doi.org/10.1371/journal.pone.0250123.s007
S6 Table. Summary of news sources.
https://doi.org/10.1371/journal.pone.0250123.s008
- View Article
- Google Scholar
- 2. World Health Organization. The Power of Vaccines: Still not fully utilized. WHO; 2020. https://www.who.int/publications/10-year-review/vaccines/en/ .
- 4. Immunization Safety Review Committee. Immunization safety review: vaccines and autism. National Academies Press; 2004 Sep 30.
- PubMed/NCBI
- 25. Luton R, Hare C. Conservatives are more likely to believe that vaccines cause autism. The Washington Post. 2015 March 1. https://www.washingtonpost.com/news/monkey-cage/wp/2015/03/01/conservatives-are-more-likely-to-believe-that-vaccines-cause-autism/ .
- 26. Reinhart R. Fewer in US continue to see vaccines as important. 2020 Jan 24. https://news.gallup.com/poll/276929/fewer-continue-vaccines-important.aspx .
- 28. Summers, Juana Timeline: How Trump Has Downplayed The Coronavirus Pandemic. NPR. 2020 October 2, 2020. https://www.npr.org/sections/latest-updates-trump-covid-19-results/2020/10/02/919432383/how-trump-has-downplayed-the-coronavirus-pandemic
- 29. Ortiz J, Hauck G. Coronavirus in the US. USA Today. 2020 March 30, 2020. https://www.usatoday.com/story/news/nation/2020/03/30/coronavirus-stay-home-shelter-in-place-orders-by-state/5092413002/ .
- 30. Elflein J. State of Health. World Health Organization. 2020 October 5. https://www.statista.com/statistics/1103185/cumulative-coronavirus-covid19-cases-number-us-by-day/ .
- 31. United States Census Bureau. Quick Facts. 2019. https://www.census.gov/quickfacts/fact/table/US/PST045219 .
- 32. Smith TW, Davern M, Freese J, Morgan S. General Social Surveys, National Science Foundation. 2020. gssdataexplorer.norc.org
- 34. Wooldridge JM. Econometric analysis of cross section and panel data. MIT press; 2010 Oct 1.
- 46. Anderson J. She Hunts Viral Rumors about Real Viruses. The New York Times. 2020 October 13. https://www.nytimes.com/2020/10/13/health/coronavirus-vaccine-hesitancy-larson.html .
- 47. Bump P. Coronavirus has come to Trump country. The Washington Post. 2020 June 17. https://www.washingtonpost.com/politics/2020/06/17/coronavirus-has-come-trump-country/ .
- 48. Quinn M. Fauci warns U.S. "unlikely" to reach Herd Immunity if too Many Refuse Vaccine. CBS News. 2020 June 29. https://www.cbsnews.com/news/fauci-herd-immunity-coronavirus-vaccine/ .
- 49. World Health Organization. Improving vaccination demand and addressing hesitancy. WHO; 2019. [cited 2020 November 3]. https://www.who.int/immunization/programmes_systems/vaccine_hesitancy/en/ .
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
April 9, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
Write it down, then throw it away: Research confirms a simple method for reducing anger
by Nagoya University

A research group in Japan has discovered that writing down one's reaction to a negative incident on a piece of paper and then shredding it or throwing it away reduces feelings of anger.
"We expected that our method would suppress anger to some extent," lead researcher Nobuyuki Kawai said. "However, we were amazed that anger was eliminated almost entirely."
This research is important because controlling anger at home and in the workplace can reduce negative consequences in our jobs and personal lives. Unfortunately, many anger management techniques proposed by specialists lack empirical research support. They can also be difficult to recall when angry.
The results of this study, published in Scientific Reports , are the culmination of years of previous research on the association between the written word and anger reduction. It builds on work showing how interactions with physical objects can control a person's mood.
For their project, Kawai and his graduate student Yuta Kanaya, both at the Graduate School of Informatics, Nagoya University, asked participants to write brief opinions about important social problems, such as whether smoking in public should be outlawed. They then told them that a doctoral student at Nagoya University would evaluate their writing.
However, the doctoral students doing the evaluation were plants. Regardless of what the participants wrote, the evaluators scored them low on intelligence, interest, friendliness, logic, and rationality. To really drive home the point, the doctoral students also wrote the same insulting comment: "I cannot believe an educated person would think like this. I hope this person learns something while at the university."
After handing out these negative comments , the researchers asked the participants to write their thoughts on the feedback, focusing on what triggered their emotions. Finally, one group of participants was told to either dispose of the paper they wrote in a trash can or keep it in a file on their desk. A second group was told to destroy the document in a shredder or put it in a plastic box.
The students were then asked to rate their anger after the insult and after either disposing of or keeping the paper. As expected, all participants reported a higher level of anger after receiving insulting comments. However, the anger levels of the individuals who discarded their paper in the trash can or shredded it returned to their initial state after disposing of the paper. Meanwhile, the participants who held on to a hard copy of the insult experienced only a small decrease in their overall anger.
Kawai imagines using his research to help businesspeople who find themselves in stressful situations. "This technique could be applied in the moment by writing down the source of anger as if taking a memo and then throwing it away when one feels angry in a business situation," he explained.
Along with its practical benefits, this discovery may shed light on the origins of the Japanese cultural tradition known as "hakidashisara" ("hakidashi" refers to the purging or spitting out of something, and "sara" refers to a dish or plate) at the Hiyoshi shrine in Kiyosu, Aichi Prefecture, just outside of Nagoya. Hakidashisara is an annual festival where people smash small disks representing things that make them angry. Their findings may explain the feeling of relief that participants report after leaving the festival.
Explore further
Feedback to editors

AI makes retinal imaging 100 times faster, compared to manual method
3 hours ago
Study highlights impact of aldehydes on DNA damage and aging
Connecting lab-grown brain cells provides insight into how our own brains work

Study leverages artificial intelligence to predict risk of bedsores in hospitalized patients
4 hours ago

Popular diabetes drugs do not increase thyroid cancer risk, study suggests
13 hours ago

Job insecurity in early adulthood linked to heightened risk of serious alcohol-related illness in later life
14 hours ago

Cognitive decline may be detected using network analysis, according to researchers
16 hours ago
Morphine tolerance found to result from Tiam1-mediated maladaptive plasticity in spinal neurons

Study finds that efforts to help low-income Americans by buying up their medical debt aren't going as planned

Mouse study uncovers how altered gene expression can induce autism
Related stories.

Want to achieve your goals? Get angry, say researchers
Oct 30, 2023

Breathe, don't vent: Turning down the heat is key to managing anger, study suggests
Mar 18, 2024

Expressing workplace anger: Not the way to get ahead, says study
Feb 20, 2024

The older people think a Black child is, the more likely they are to wrongly see the child as angry
Aug 5, 2021

Using virtual reality for anger control
May 9, 2022

Angry people might not be as smart as they think they are
Aug 13, 2018
Recommended for you
How childhood stress influences gene activity and increases the risk of mental illness
22 hours ago

Exploring how oxytocin interacts with testosterone while humans play a game modeling intergroup conflict
Apr 8, 2024
Heart disease and depression may be genetically linked by inflammation

Everyday social interactions predict language development in infants

New study highlights the benefit of touch on mental and physical health

Youths with mood disorders 30% less likely to acquire driver's license than peers, researchers find
Let us know if there is a problem with our content.
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.

Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
A Discrimination Report Card
We develop an empirical Bayes ranking procedure that assigns ordinal grades to noisy measurements, balancing the information content of the assigned grades against the expected frequency of ranking errors. Applying the method to a massive correspondence experiment, we grade the race and gender contact gaps of 97 U.S. employers, the identities of which we disclose for the first time. The grades are presented alongside measures of uncertainty about each firm’s contact gap in an accessible report card that is easily adaptable to other settings where ranks and levels are of simultaneous interest.
We thank Ben Scuderi for helpful feedback on an early draft of this paper and Hadar Avivi and Luca Adorni for outstanding research assistance. Seminar participants at Brown University, the 2022 California Econometrics Conference, Columbia University, CIREQ 2022 Montreal, Harvard University, Microsoft Research, Monash University, Peking University, Royal Holloway, UC Santa Barbara, UC Berkeley, The University of Virginia, the Cowles Econometrics Conference on Discrimination and Algorithmic Fairness, and The University of Chicago Interactions Conference provided useful comments. Routines for implementing the ranking procedures developed in this paper are available online at https://github.com/ekrose/drrank. The views expressed herein are those of the authors and do not necessarily reflect the views of the National Bureau of Economic Research.
Christopher Walters holds concurrent appointments as an Associate Professor of Economics at UC Berkeley and as an Amazon Scholar. This paper describes work performed at UC Berkeley and is not associated with Amazon.
MARC RIS BibTeΧ
Download Citation Data
- randomized controlled trials registry entry
- GitHub archive
Working Groups
Conferences, mentioned in the news, more from nber.
In addition to working papers , the NBER disseminates affiliates’ latest findings through a range of free periodicals — the NBER Reporter , the NBER Digest , the Bulletin on Retirement and Disability , the Bulletin on Health , and the Bulletin on Entrepreneurship — as well as online conference reports , video lectures , and interviews .

Read our research on: Gun Policy | International Conflict | Election 2024
Regions & Countries
6 facts about americans and tiktok.
Increasing shares of U.S. adults are turning to the short-form video sharing platform TikTok in general and for news .
Pew Research Center conducted this analysis to better understand Americans’ use and perceptions of TikTok. The data for this analysis comes from several Center surveys conducted in 2023.
More information about the surveys and their methodologies, including the sample sizes and field dates, can be found at the links in the text.
Pew Research Center is a subsidiary of The Pew Charitable Trusts, its primary funder. This is the latest analysis in Pew Research Center’s ongoing investigation of the state of news, information and journalism in the digital age, a research program funded by The Pew Charitable Trusts, with generous support from the John S. and James L. Knight Foundation.
This analysis draws from several Pew Research Center reports on Americans’ use of and attitudes about social media, based on surveys conducted in 2023. For more information, read:
Americans’ Social Media Use
How u.s. adults use tiktok.
- Social Media and News Fact Sheet
- Teens, Social Media and Technology 2023
At the same time, some Americans have concerns about the Chinese-owned platform’s approach to data privacy and its potential impact on national security. Lawmakers in the U.S. House of Representatives recently passed a bill that, if passed in the Senate and signed into law, would restrict TikTok’s ability to operate in the United States.
Here are six key facts about Americans and TikTok, drawn from Pew Research Center surveys.
A third of U.S. adults – including a majority of adults under 30 – use TikTok. Around six-in-ten U.S. adults under 30 (62%) say they use TikTok, compared with 39% of those ages 30 to 49, 24% of those 50 to 64, and 10% of those 65 and older.
In a 2023 Center survey , TikTok stood out from other platforms we asked about for the rapid growth of its user base. Just two years earlier, 21% of U.S. adults used the platform.
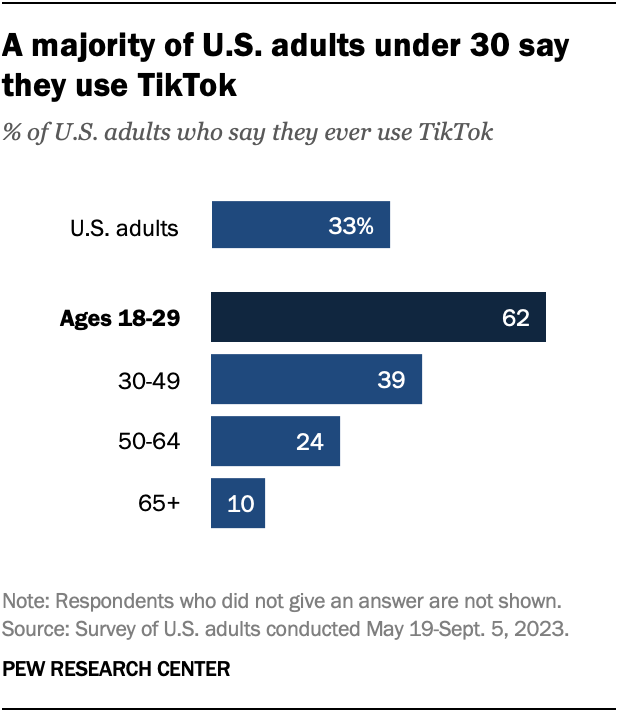
A majority of U.S. teens use TikTok. About six-in-ten teens ages 13 to 17 (63%) say they use the platform. More than half of teens (58%) use it daily, including 17% who say they’re on it “almost constantly.”
A higher share of teen girls than teen boys say they use TikTok almost constantly (22% vs. 12%). Hispanic teens also stand out: Around a third (32%) say they’re on TikTok almost constantly, compared with 20% of Black teens and 10% of White teens.
In fall 2023, support for a U.S. TikTok ban had declined. Around four-in-ten Americans (38%) said that they would support the U.S. government banning TikTok, down from 50% in March 2023. A slightly smaller share (27%) said they would oppose a ban, while 35% were not sure. This question was asked before the House of Representatives passed the bill that could ban the app.
Republicans and Republican-leaning independents were far more likely than Democrats and Democratic leaners to support a TikTok ban (50% vs. 29%), but support had declined across both parties since earlier in the year.
Adults under 30 were less likely to support a ban than their older counterparts. About three-in-ten adults under 30 (29%) supported a ban, compared with 36% of those ages 30 to 49, 39% of those ages 50 to 64, and 49% of those ages 65 and older.
In a separate fall 2023 survey, only 18% of U.S. teens said they supported a ban.
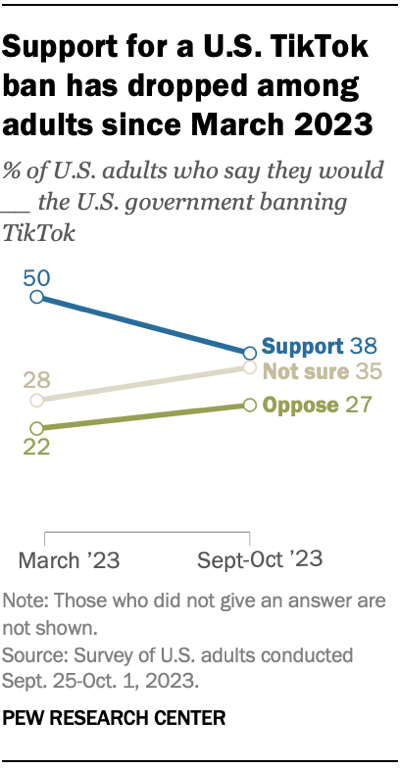
A relatively small share of users produce most of TikTok’s content. About half of U.S. adult TikTok users (52%) have ever posted a video on the platform. In fact, of all the TikTok content posted by American adults, 98% of publicly accessible videos come from the most active 25% of users .
Those who have posted TikTok content are more active on the site overall. These users follow more accounts, have more followers and are more likely to have filled out an account bio.
Although younger U.S. adults are more likely to use TikTok, their posting behaviors don’t look much different from those of older age groups.
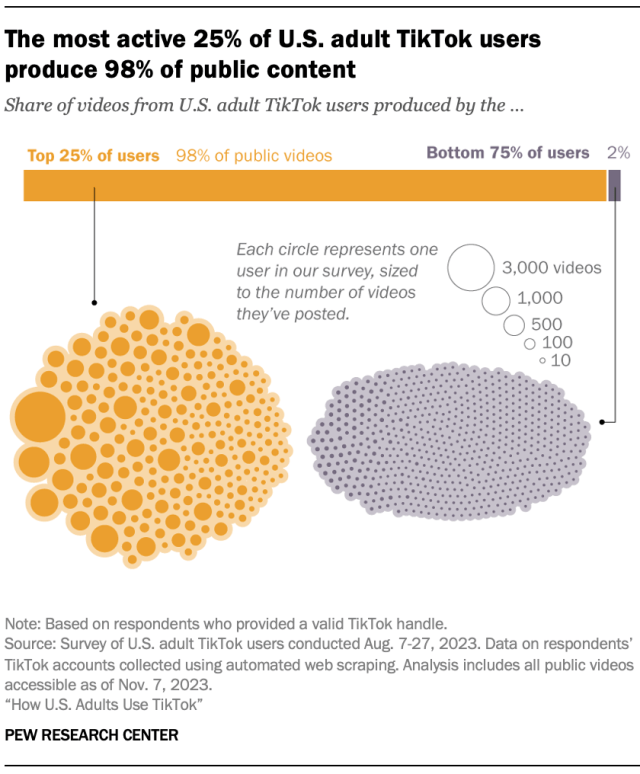
About four-in-ten U.S. TikTok users (43%) say they regularly get news there. While news consumption on other social media sites has declined or remained stagnant in recent years, the share of U.S. TikTok users who get news on the site has doubled since 2020, when 22% got news there.
Related: Social Media and News Fact Sheet
TikTok news consumers are especially likely to be:
- Young. The vast majority of U.S. adults who regularly get news on TikTok are under 50: 44% are ages 18 to 29 and 38% are 30 to 49. Just 4% of TikTok news consumers are ages 65 and older.
- Women. A majority of regular TikTok news consumers in the U.S. are women (58%), while 39% are men. These gender differences are similar to those among news consumers on Instagram and Facebook.
- Democrats. Six-in-ten regular news consumers on TikTok are Democrats or Democratic-leaning independents, while a third are Republicans or GOP leaners.
- Hispanic or Black. Three-in-ten regular TikTok news users in the U.S. are Hispanic, while 19% are Black. Both shares are higher than these groups’ share of the adult population. Around four-in-ten (39%) TikTok news consumers are White, although this group makes up 59% of U.S. adults overall .
A majority of Americans (59%) see TikTok as a major or minor threat to U.S. national security, including 29% who see the app as a major threat. Our May 2023 survey also found that opinions vary across several groups:
- About four-in-ten Republicans (41%) see TikTok as a major threat to national security, compared with 19% of Democrats.
- Older adults are more likely to see TikTok as a major threat: 46% of Americans ages 65 and older say this, compared with 13% of those ages 18 to 29.
- U.S. adults who do not use TikTok are far more likely than TikTok users to believe TikTok is a major threat (36% vs. 9%).
Sign up for our weekly newsletter
Fresh data delivered Saturday mornings
WhatsApp and Facebook dominate the social media landscape in middle-income nations
Teens and social media fact sheet, most popular.
About Pew Research Center Pew Research Center is a nonpartisan fact tank that informs the public about the issues, attitudes and trends shaping the world. It conducts public opinion polling, demographic research, media content analysis and other empirical social science research. Pew Research Center does not take policy positions. It is a subsidiary of The Pew Charitable Trusts .
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 22 August 2022
A systematic literature review to clarify the concept of vaccine hesitancy
- Daphne Bussink-Voorend ORCID: orcid.org/0000-0002-9873-1404 1 ,
- Jeannine L. A. Hautvast 1 ,
- Lisa Vandeberg ORCID: orcid.org/0000-0002-7229-2378 2 ,
- Olga Visser 1 &
- Marlies E. J. L. Hulscher ORCID: orcid.org/0000-0002-2160-4810 3
Nature Human Behaviour volume 6 , pages 1634–1648 ( 2022 ) Cite this article
16k Accesses
60 Citations
53 Altmetric
Metrics details
- Human behaviour
- Infectious diseases
- Preventive medicine
Vaccine hesitancy (VH) is considered a top-10 global health threat. The concept of VH has been described and applied inconsistently. This systematic review aims to clarify VH by analysing how it is operationalized. We searched PubMed, Embase and PsycINFO databases on 14 January 2022. We selected 422 studies containing operationalizations of VH for inclusion. One limitation is that studies of lower quality were not excluded. Our qualitative analysis reveals that VH is conceptualized as involving (1) cognitions or affect, (2) behaviour and (3) decision making. A wide variety of methods have been used to measure VH. Our findings indicate the varied and confusing use of the term VH, leading to an impracticable concept. We propose that VH should be defined as a state of indecisiveness regarding a vaccination decision.
Similar content being viewed by others

Long COVID: major findings, mechanisms and recommendations
Hannah E. Davis, Lisa McCorkell, … Eric J. Topol

Increase in concerns about climate change following climate strikes and civil disobedience in Germany
Johannes Brehm & Henri Gruhl

A guide to vaccinology: from basic principles to new developments
Andrew J. Pollard & Else M. Bijker
In 2019, vaccine hesitancy (VH) was named by the World Health Organization (WHO) as one of the top-10 threats to global health, following a five-fold global increase in measles, a disease that can be prevented by vaccination 1 , 2 . The largest increase was reported in the WHO regions covering Europe and the Americas 2 . The impact of these measles outbreaks is substantial, with rises in morbidity, mortality and costs 3 , 4 , 5 . The increasing incidence of measles and other vaccine-preventable diseases has been attributed to a failure to reach adequate immunization coverage rates 2 , 6 . In the European region, VH has been identified as the main barrier to vaccination coverage 7 , 8 . This is in contrast to other regions, such as sub-Saharan Africa, where immunization coverage rates are challenged by a combination of barriers, including access and availability 9 .
In the past decade, VH has become a key topic of research in various fields, following rises in vaccine-preventable diseases, the introduction of new vaccines, the spread of misinformation and lagging vaccination coverage 10 . Moreover, the COVID-19 pandemic has drawn further attention to the role of VH in limiting the uptake of vaccines and failure to achieve collective immunity 11 , 12 , 13 . This has led to the proliferation of scientific literature on VH in the public health, biomedical and social science research fields 10 .
In 2012, the WHO established a strategic advisory group of experts (SAGE) working group with the mandate of defining VH and suggesting how to monitor and address it. The working group proposed a broad definition, describing a VH continuum from acceptance to refusal of vaccines or as a delay in acceptance or refusal despite the availability of the vaccines. The working group described VH as “A complex behavioural phenomenon specific to vaccines, context, time, and place and influenced by factors of complacency, convenience, and confidence” 14 . This broad definition emphasizes variability by describing that VH may vary between types of vaccines and different contexts, may change over time or between different geographical locations and is influenced by various determinants.
The concept of VH has been described and applied in various ways. When definitions are broad and lack clarity, this can lead to the emergence of different concepts with overlapping domains, with various concepts being used interchangeably by some and recognized as distinct entities by others 15 . Additionally, lack of conceptual clarity can lead to inadequate operationalization and cause confusion among researchers 15 . This is problematic because when studies use similar terminology with a different meaning, their results are incomparable across subgroups, locations or contexts. A clear conceptualization is needed to develop meaningful measures allowing comparison of results 16 .
A lack of conceptual clarity is observed in the literature on VH, where VH is variously conceptualized as a psychological state and as different types of vaccination behaviour 17 , 18 . In addition, the terms ‘vaccine confidence’, ‘low uptake’ and ‘low intention to vaccinate’ are often equated with VH 19 , 20 . Confusion among researchers is then illustrated by inconsistencies in the applied definitions 21 , 22 . It has even been argued that VH is a catch-all category, aggregating many different concepts rather than being one measurable construct; and this is impeding progress in the research field 23 .
A good concept definition consists of characteristics, attributes or features that are unique to that concept and distinguish it from other closely related concepts 15 . Given the importance of VH for predicting and influencing individual vaccination decisions, it is important to explore the uses of VH and propose an optimal operationalization, distinguishing VH from other closely related concepts. Such clarification could enable a universally adopted definition and aid further research in this area.
The purpose of this systematic review was to provide an overview of how VH is operationalized in the literature in terms of conceptualizations, subpopulations and measurements. Following an assessment of the various conceptualizations, we differentiated the common themes, related concepts, research fields and vaccine types. The scope and structure of this systematic review is visualized in Fig. 1 . On the basis of an interpretation of these findings, we suggest a way forward by proposing a renewed definition for VH.
Aiming to give an overview of VH, we recognize three types of operationalizations: conceptualizations (blue), identification of subpopulations (orange) and measurements (green). Conceptualizations of VH are analysed at three levels: (1) common themes, (2) closely related concepts and (3) potential variation in conceptualization between research field and vaccine type. Each type of operationalization and its levels are discussed in separate sections.
Study selection and characteristics
The search strategy yielded 7,427 publications. After screening the titles and abstracts, 919 publications were selected for full-text screening. A total of 420 publications met the inclusion criteria. Seven additional studies were found through citation searching, two of which met the inclusion criteria, adding up to a total of 422 studies. Some studies met the criteria of more than one category, with 36 studies categorized under VH conceptualizations, 63 under VH subpopulations and 373 under VH measurements. The search process is summarized in the PRISMA flow chart (Fig. 2 ) 24 . The characteristics of included studies are described in more detail in Supplementary Table 1 .
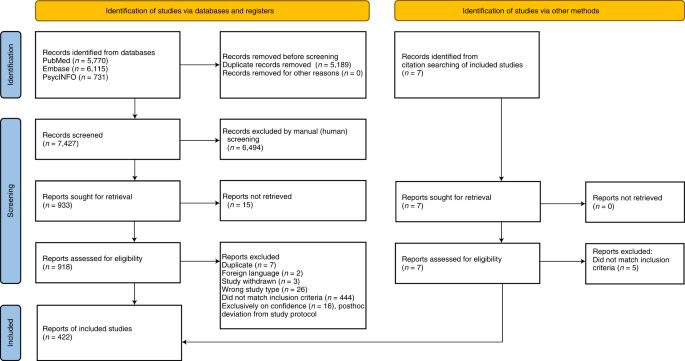
Visualization of the process involving identification of records from databases, screening of records, assessing reports for eligibility, inclusion of eligible studies and exclusion of non-eligible reports with reasons for exclusion. The number of records or reports in each step of the process is shown in brackets.
The included studies cover a wide geographical distribution. The limited majority (54%) originated in high-income countries (HIC), mainly the United States, Canada, Italy, Australia and France. A smaller group (43%) originated in low- and middle-income countries (LMICs), primarily China, India and Turkey. The remaining studies (3%) originated in a combination of HIC and LMICs. The majority (60%) were published in 2021 and 2022.
The included studies approach VH in relation to various vaccine types: 51% pertaining to COVID-19, 29% to childhood, 4% to human papillomavirus, 4% to influenza and 2% to miscellaneous vaccines. Additionally, 11% of the studies concern vaccines in general. Various research fields are represented, including public health (43%), biomedical science (30%), paediatrics (15%) and social sciences (12%). Mixed methods appraisal tool (MMAT) scores were calculated for 88% of the included studies, while the others could not be assessed due to their study types. The majority (68%) scored 3 or higher, indicating that 60% of the quality criteria were met.
Vaccine hesitancy conceptualization
From the 36 studies on VH conceptualization, we extracted and analysed 304 excerpts. Supplementary Table 2 shows the extracted text excerpts for each study. Our thematic analysis revealed that 93 excerpts describe an overall characterization of VH. The majority of these (69%) describe the nature of VH as heterogenous 14 , 21 , 23 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , complex 14 , 18 , 20 , 21 , 22 , 23 , 25 , 26 , 29 , 33 , 35 , 38 , 39 , 40 , 41 , 42 , 43 or varied, depending on the type of vaccine and the context 14 , 18 , 20 , 21 , 23 , 27 , 28 , 30 , 33 , 35 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 .
VH is conceptualized in 208 excerpts. The thematic analysis revealed three predominant conceptualizations in 165 (79%) excerpts: cognitions or affect, behaviour and decision making. These three conceptualizations overlap in the majority of the studies and excerpts. Illustrative excerpts of each conceptualization are presented in Table 1 . The remaining 45 (22%) excerpts represent a fragmented group of conceptualizations, without emerging themes.
Vaccine hesitancy conceptualized as cognitions or affect
From all 36 studies 14 , 17 , 18 , 20 , 21 , 22 , 23 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 98 excerpts were extracted as conceptualizing VH in terms of cognitions or affect, including questioning, emotions or beliefs regarding vaccination. For this conceptualization, we rank-ordered the most frequently used descriptions of VH, including having or expressing concerns 21 , 25 , 26 , 27 , 29 , 30 , 34 , 35 , 36 , 40 , 42 , 43 , 46 , 51 , 53 , doubts 21 , 28 , 29 , 36 , 43 or questions 21 , 26 , 47 and being reluctant 23 , 27 , 29 , 32 , 36 , 38 , 45 , 49 , 53 , 54 or unsure 14 , 21 , 27 , 29 , 34 . Many authors describe VH as pertaining to beliefs 34 , 49 , attitudes 21 , 26 , 37 , 43 , 51 or both 23 , 29 , 30 , 55 . Furthermore, vaccine-hesitant individuals are described as ambivalent to vaccination or perceiving ambiguity in vaccine-related risks 21 , 36 , 50 , 53 .
Vaccine hesitancy conceptualized as behaviour
From 35 studies 14 , 17 , 18 , 20 , 21 , 22 , 23 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 94 excerpts were extracted as conceptualizing VH as a behaviour. The majority of the excerpts describe VH in terms of various behaviours 14 , 18 , 20 , 21 , 22 , 23 , 25 , 26 , 27 , 29 , 31 , 32 , 34 , 35 , 37 , 38 , 39 , 40 , 41 , 44 , 45 , 51 , as illustrated by the following example: “VH refers to a ‘delay’ in acceptance or ‘refusal’ of vaccines” 14 . Other excerpts describe VH as a range or continuum between the extreme ends of accepting all vaccines and refusing all vaccines 21 , 22 , 27 , 28 , 29 , 30 , 31 , 33 , 36 , 38 , 43 . In a minority of the excerpts, VH is described as a specific type of vaccination behaviour, including vaccinating as recommended (despite reluctance, concerns or feeling unsure) 26 , 46 , 47 , 49 , refusing vaccines 28 or delaying vaccines and choosing an alternative schedule 50 . Some studies explicitly state that VH should not be described as a vaccination behaviour 17 , 18 , 36 , 40 . Within articles, there were inconsistencies in the behavioural descriptions of VH 18 , 22 , 26 , 27 , 28 , 29 , 31 , 38 , 41 .
Vaccine hesitancy conceptualized as decision making
From 19 studies 18 , 21 , 23 , 26 , 27 , 30 , 31 , 32 , 36 , 37 , 38 , 40 , 42 , 44 , 45 , 50 , 52 , 53 , 30 excerpts were extracted as conceptualizing VH in terms of vaccine decision-making. Some authors adopt the term VH when describing individuals who are undecided, indecisive or under consideration, and not yet having made a final vaccine decision 21 , 23 , 26 , 31 , 32 , 45 , 50 . Vaccine-hesitant individuals are described as being in various states of indecision 23 , 31 , 32 , 37 or as seeking more information to make ‘the right decision’ about vaccination 21 , 53 . Moreover, some authors describe VH as an approach to 38 or a transient stage in the process of vaccine decision-making itself 21 , 23 , 37 .
Vaccine hesitancy and related concepts
VH is often described in relation to other concepts. We extracted 142 excerpts from 31 studies describing closely related concepts 14 , 18 , 20 , 21 , 22 , 23 , 25 , 26 , 27 , 29 , 30 , 32 , 33 , 34 , 35 , 36 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 50 , 51 , 52 , 53 . The three most common concepts are confidence or trust, complacency and convenience. Together, these are referred to as ‘the 3 Cs’ 14 and described in 69 of 142 (49%) excerpts. Most often, the 3 Cs are described as having a causal relationship with VH and as representing determinants 14 , 18 , 20 , 29 , 33 , 35 , 38 , 41 , 48 , 56 .
From 25 studies, 46 excerpts were extracted as describing confidence 14 , 18 , 20 , 21 , 22 , 23 , 25 , 26 , 27 , 29 , 30 , 33 , 34 , 35 , 36 , 38 , 39 , 41 , 42 , 43 , 44 , 46 , 47 , 48 , 52 . ‘Confidence’ is defined as the trust that people have in the immunizations, the healthcare system itself, and the process leading to decisions on licensing or recommended schedules 14 , 27 , 35 . Few studies describe the (lack of) trust or confidence as a component of VH 23 , 34 , 52 .
From 22 studies 14 , 18 , 20 , 21 , 22 , 23 , 25 , 26 , 29 , 30 , 33 , 35 , 38 , 39 , 40 , 41 , 43 , 44 , 47 , 48 , 50 , 52 , 41 excerpts were extracted on the theme of complacency. ‘Complacency’ is the individual evaluation of the risks and benefits of vaccines and of the need to vaccinate 14 , 18 , 20 , 35 . The concept of complacency in relation to VH is described as the tendency to perceive the risks of vaccination as unknown or disproportionally high and the risks of the vaccine-preventable disease as low 44 , 50 . Vaccine-hesitant individuals are more committed to assessing vaccine risks and seeking ways to minimize them 23 , 40 , 47 , 50 .
From 15 studies 14 , 18 , 20 , 21 , 22 , 25 , 29 , 33 , 35 , 38 , 39 , 41 , 42 , 43 , 48 , 27 excerpts were extracted as describing the theme of convenience. ‘Convenience’ concerns not only physical availability and geographical accessibility of vaccines, but also the user-friendliness of and ability to understand immunization services 14 , 18 , 35 , 42 . In our analysis, we found that many authors refer to convenience by describing VH as the delaying or refusal of vaccines ‘despite availability’ 14 , 18 , 21 , 22 , 23 , 25 , 26 , 29 , 33 , 35 , 38 , 39 , 41 . This description acknowledges that availability of vaccines is related to vaccine uptake, while VH itself is not influenced by availability issues. However, one study adopts inconvenience and difficulty to access vaccines as dimensions of VH 42 .
Variations between research fields and vaccine types
We identified the respective research field and vaccine type of each study in the qualitative analysis to explore related differences in descriptions of VH. We identified 19 public health studies 18 , 21 , 23 , 25 , 26 , 27 , 28 , 29 , 32 , 33 , 36 , 37 , 38 , 41 , 45 , 47 , 50 , 51 , 53 , 6 paediatric studies 14 , 31 , 34 , 35 , 39 , 48 , 8 social science studies 17 , 20 , 22 , 42 , 44 , 46 , 49 , 52 and 3 biomedical studies 30 , 40 , 43 . The primary difference observed was that conceptualizations of VH in terms of decision making emerged predominantly in the public health 18 , 21 , 23 , 32 , 38 , 50 , 54 and social science fields 42 , 44 , 52 . In studies conceptualizing VH in terms of cognitions or affect, the terms ‘beliefs’ and ‘concerns’ were used in all research fields, while ‘reluctance’, ‘doubts’ and ‘questions’ were used almost exclusively in the public health field. The conceptualization of VH as a behaviour occurred in all research fields.
VH was discussed in relation to vaccination in general 14 , 17 , 18 , 22 , 23 , 27 , 28 , 29 , 32 , 33 , 35 , 36 , 38 , 41 , 42 , 43 , 46 , 48 , 49 or specifically with regard to childhood vaccines 21 , 25 , 26 , 30 , 31 , 34 , 37 , 39 , 40 , 47 , 50 , 51 , 53 , in 19 and 13 of the studies, respectively. The remaining 4 studies discussed VH in relation to COVID-19 44 , 45 , 52 and influenza 20 . Our analysis compared the studies on general vaccination and childhood vaccines but found no major differences in their respective conceptualizations.
Vaccine hesitancy subpopulations
Of the 422 included studies, 63 identified various VH subpopulations. We extracted text excerpts describing the classifications of these subpopulations and the authors’ rationales for the distinctions. The analysis identified themes aligned with the three VH conceptualization categories. Fourteen studies grouped VH subpopulations on the basis of criteria from the conceptualization as cognitions or affect 21 , 23 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 and 3 studies grouped VH on the basis of the conceptualization of decision making 69 , 70 , 71 . VH subpopulations grouped solely on the basis of criteria from the behaviour conceptualization were not found. However, 19 studies grouped hesitant individuals on the basis of criteria from the conceptualizations of both cognitions or affect, and behaviour 26 , 47 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 . The remaining 27 studies did not identify subpopulations in terms of the three conceptualizations. Twelve studies identified subpopulations on the basis of degree of VH 51 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 . Although degree of VH does not directly contribute to understanding of the VH concept, the instruments used to quantify it and determine cut-off values for the subpopulations contain valuable information about the operationalizations. These instruments are discussed in the following section. In addition, a group of 10 studies distinguished a VH subpopulation by asking about willingness to be vaccinated but used different criteria to do so 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 . This method was mainly found in studies on COVID-19 vaccination, published in 2021. This demonstrates the emergence of a conceptual VH category that was not identified from the conceptual studies. The final 5 studies grouped subpopulations according to miscellaneous criteria 45 , 49 , 110 , 111 , 112 . An overview is provided Supplementary Table 3 .
Measurements of vaccine hesitancy
Of the 422 studies included, 373 report a measurement of VH in individuals. An overview is provided in Supplementary Table 4 , grouping the studies according to the instruments used. The most common, albeit highly heterogenous, method used in 210 (56%) studies is a brief VH assessment comprising 1–3 questions 64 , 65 , 66 , 68 , 71 , 74 , 75 , 84 , 85 , 88 , 90 , 96 , 97 , 98 , 100 , 102 , 103 , 105 , 106 , 107 , 108 , 109 , 111 , 113 , 114 , 115 , 116 , 117 , 118 , 119 , 120 , 121 , 122 , 123 , 124 , 125 , 126 , 127 , 128 , 129 , 130 , 131 , 132 , 133 , 134 , 135 , 136 , 137 , 138 , 139 , 140 , 141 , 142 , 143 , 144 , 145 , 146 , 147 , 148 , 149 , 150 , 151 , 152 , 153 , 154 , 155 , 156 , 157 , 158 , 159 , 160 , 161 , 162 , 163 , 164 , 165 , 166 , 167 , 168 , 169 , 170 , 171 , 172 , 173 , 174 , 175 , 176 , 177 , 178 , 179 , 180 , 181 , 182 , 183 , 184 , 185 , 186 , 187 , 188 , 189 , 190 , 191 , 192 , 193 , 194 , 195 , 196 , 197 , 198 , 199 , 200 , 201 , 202 , 203 , 204 , 205 , 206 , 207 , 208 , 209 , 210 , 211 , 212 , 213 , 214 , 215 , 216 , 217 , 218 , 219 , 220 , 221 , 222 , 223 , 224 , 225 , 226 , 227 , 228 , 229 , 230 , 231 , 232 , 233 , 234 , 235 , 236 , 237 , 238 , 239 , 240 , 241 , 242 , 243 , 244 , 245 , 246 , 247 , 248 , 249 , 250 , 251 , 252 , 253 , 254 , 255 , 256 , 257 , 258 , 259 , 260 , 261 , 262 , 263 , 264 , 265 , 266 , 267 , 268 , 269 , 270 , 271 , 272 , 273 , 274 , 275 , 276 , 277 , 278 , 279 , 280 , 281 , 282 , 283 , 284 , 285 , 286 , 287 , 288 , 289 , 290 , 291 , 292 , 293 , 294 , 295 , 296 , 297 , 298 . The questions, as well as the criteria or cut-off points used to define hesitancy, vary widely between the studies. The majority of questions used in this method cover operationalizations of VH that did not emerge from our conceptual analysis, including intention and willingness. A group of 124 studies assess VH by asking about vaccination intention. For example, one measurement asks “What would you do if a COVID-19 vaccine were available?”. Respondents answering either “I would eventually get a vaccine, but wait a while first”, “I would not get a vaccine” or “I’m not sure” are all classified as hesitant 169 . A group of 35 studies assess VH by asking about willingness, exemplified by the question: “Are you willing to receive the COVID-19 vaccination?”. Respondents answering “yes, but I choose to delay timing of injection” are considered hesitant 100 . Furthermore, 23 studies assess VH by an explicit verbatim assessment of experienced hesitancy levels. This is exemplified by the question: “Overall, how hesitant about childhood vaccines would you consider yourself to be?”. Respondents answering “not too hesitant”, “not sure”, “somewhat hesitant” or “very hesitant” are considered hesitant 136 . Finally, a minority of 14 studies assess VH with questions covering conceptualizations that did emerge from our conceptual analysis; for example, by asking about previous vaccination behaviour: “Have you ever hesitated, delayed, or refused getting a vaccination for your child or yourself due to reasons other than allergies and sickness?”. Respondents answering “yes” to this question are considered hesitant 122 . The remaining 14 studies use miscellaneous questions to assess VH. Notably, the intention and willingness measures to assess VH are found mainly in studies published in 2021 on COVID-19 vaccination, while the other methods have been used throughout the covered period and in the context of different vaccines.
The second most common method, applied by 132 (35%) studies, is the use of a validated instrument. The most common instrument, used in 70 studies, is the parent attitudes about childhood vaccines (PACV) survey, introduced by Opel et al. 34 . The PACV consists of 15 questions about immunization behaviour, beliefs about vaccine safety and efficacy, attitudes toward vaccine mandates and exemptions, and trust 299 , thereby operationalizing VH as both cognitions or affect, and behaviour. Trust (or confidence) is also included in this instrument. In our conceptual analysis, confidence emerged as a distinct concept, albeit closely related to VH. Clear cut-off points for hesitancy were formulated and applied in the vast majority of the studies using this instrument (shown in Supplementary Table 4 ). The PACV is variously used in its original form 34 , 91 , 299 , 300 , 301 , 302 , 303 , 304 , 305 , 306 , 307 , 308 , 309 , 310 , 311 , 312 , 313 , 314 , 315 , 316 , 317 , 318 , 319 , 320 , 321 , 322 , 323 , 324 , 325 , 326 , 327 , 328 , 329 , 330 , 331 , 332 , 333 , 334 , 335 , 336 , 337 , 338 , or in adapted 339 , 340 , 341 , 342 , 343 , 344 , 345 , 346 , 347 , 348 , 349 , 350 , 351 , 352 , 353 , 354 , 355 or shorter versions 51 , 62 , 89 , 93 , 95 , 356 , 357 , 358 , 359 , 360 , 361 .
Other studies use a variety of validated and broadly used instruments. The SAGE instrument is applied in 13 of the studies 41 , 362 , 363 , 364 , 365 , 366 , 367 , 368 , 369 , 370 , 371 , 372 , 373 , with questions reflecting the different conceptualizations (cognitions or affect, behaviour and decision making) and related concepts including convenience, complacency and confidence 41 . The vaccine hesitancy scale (VHS), used in 39 studies 83 , 99 , 374 , 375 , 376 , 377 , 378 , 379 , 380 , 381 , 382 , 383 , 384 , 385 , 386 , 387 , 388 , 389 , 390 , 391 , 392 , 393 , 394 , 395 , 396 , 397 , 398 , 399 , 400 , 401 , 402 , 403 , 404 , 405 , 406 , 407 , 408 , 409 , 410 , was derived from a subscale of the SAGE instrument, narrowed to conceptualize VH as cognitions or affect and include the related concept of confidence 69 . The studies using the SAGE instrument and VHS use varying outcomes or cut-off values (or no outcomes or cut-off values at all) to define hesitancy (shown in Supplementary Table 4 ). The Oxford COVID-19 vaccine hesitancy scale was recently designed exclusively for the assessment of VH for COVID-19 vaccination and subsequently applied in 5 studies 44 , 411 , 412 , 413 , 414 . Other instruments described in the context of VH but intended to assess other concepts include the 5C scale 22 of psychological antecedents of vaccine behaviour, the vaccine acceptance scale (which covers the domains cognitions and affects, confidence and legitimacy of government vaccine mandates 46 ) and the multidimensional vaccine hesitancy scale covering perceptions regarding vaccines in general 42 . Instruments assessing confidence have also been applied to assess hesitancy 415 .
The remaining 31 (8%) studies use a variety of unique, self-developed methods to measure hesitancy. These are classified as ‘miscellaneous’ 25 , 50 , 52 , 69 , 73 , 92 , 94 , 416 , 417 , 418 , 419 , 420 , 421 , 422 , 423 , 424 , 425 , 426 , 427 , 428 , 429 , 430 , 431 , 432 , 433 , 434 , 435 , 436 , 437 , 438 , 439 . Examples include measurement of VH based on vaccination rates from medical records 418 and statistical procedures used to group participants according to their patterned responses to a questionnaire 92 , 439 .
Our systematic review reveals that VH is conceptualized in the literature as involving cognitions or affect, behaviour and decision making, representing three distinct but interacting entities. Closely related concepts include confidence or trust, perceptions of the need to vaccinate and of risk (complacency), and convenience. VH subpopulations are grouped according to a variety of criteria, with the majority originating in the three identified conceptualizations. Studies measuring VH have used a wide variety of instruments. The most commonly applied instruments include a brief assessment comprising 1–3 variable questions and the PACV for childhood vaccines. The instruments operationalize hesitancy using one or more of the three identified conceptualizations, but also introduce novel conceptualizations including intention and willingness. When synergizing the findings on different VH operationalizations, we found psychological and behavioural operationalizations, with the psychological operationalizations being cognitions or affect, and decision making.
Our findings illustrate the challenge of operationalizing VH, with studies adopting different conceptualizations, subpopulations and measurements. Dubé et al. acknowledged this challenge of operationalizing the VH concept due to its heterogeneity and the diversity in attitudes and behaviours 29 . Furthermore, our findings align with a recent study demonstrating the many interpretations of VH used across Europe 440 . These inconsistencies in terminology are even evidenced in the Merriam-Webster dictionary, where ‘hesitancy’ is defined as a quality or state of being that involves indecision or reluctance 441 , aligning with VH conceptualized as decision making and cognitions or affect, while ‘vaccine hesitancy’ is defined as the reluctance or refusal to vaccinate 442 , thereby also including a conceptualization of behaviour.
In the introduction, we describe interchangeable use of various terms with VH 19 , 20 . In our review, we also found numerous examples, including ‘confidence’ 443 , ‘low intention’ 444 and ‘unwillingness’ 270 . We identify these concepts as related but not synonymous to VH. For instance, some authors note that confidence or trust are used interchangeably in relation to VH 19 , 22 , suggesting equivalent meanings. Others describe an inverse relationship, meaning that lower levels of confidence are associated with higher levels of VH 19 , 33 , 54 , 56 , 445 . In line with this, VH is described as originating from a lack of confidence 446 and as a possible indicator of declining confidence 56 .
Additionally, in our analysis of subgroups and measurements, we found that VH is frequently operationalized in terms of willingness and intention, which we did not find in our conceptual analysis of VH. Willingness and intention to vaccinate, similar to the ‘vaccine confidence’ concept, are inversely related concepts that are unequivocally linked to VH but are and should not be treated as synonymous. Using these terms interchangeably is not only inappropriate but also contributes to confusion and unclarity of the VH concept. This clarity is needed because unclear concepts give rise to differences in our understanding of its determinants, correlates and consequences, hindering efforts to study and address VH 15 , 23 , 440 . Furthermore, at an operational level, there may be a mismatch between a concept and its measures 15 . This is demonstrated in our review by the highly variable methods we found to measure VH, leading to incomparable results. Particularly during 2021, there has been a plethora of studies reporting VH measurements that, due to divergent definitions and methods, have been of questionable value. As a way forward, we base our reasoning for a renewed definition of VH on the three main identified conceptual categories—behaviour, cognitions or affect, and decision making—as these have proven most promising by their repeated representation in conceptual, subgroup and measurement studies
We argue that conceptualizing VH as vaccination behaviour is untenable, as mere behaviour is insufficiently discriminating between hesitant and non-hesitant individuals. For instance, people may accept vaccines with or without hesitation or reject vaccines with or without hesitation. As concepts are ideally defined by a unique set of features that distinguishes them from other closely related concepts 15 , vaccination behaviour alone is not sufficient to define VH. Furthermore, vaccination behaviour is generally used as the indicator of (non-)acceptance of vaccination. Thus, to use this also to define another concept would create confusion. Authors have commented on the blurred distinction between VH and refusal of vaccines 25 , 39 and criticized behavioural operationalization for its failure to capture VH 17 , 18 , 23 , 25 , 40 . Although we agree that certain types of vaccination behaviour may be manifestations of VH, we argue that including behaviour in the definition and operationalization of VH is neither necessary nor sufficient.
Our analysis shows that VH is furthermore defined by two closely linked conceptualizations that we identify as psychological—cognitions or affect, and decision making. Larson et al. exemplify this stance, arguing that VH is by nature a state of indecision and reluctance 32 . We propose to reject types of vaccination behaviour as a viable conceptualization of VH; this logically results in the proposition that VH should be considered a psychological construct. This is in line with authors who have argued that VH is a psychological state rather than a behaviour 18 , 22 , 26 , 32 , 40 , inspiring our current investigation of what exactly this vaccine-hesitant state entails. In the conceptualization cognitions or affect, VH is mainly described as ‘doubts’, ‘concerns’ and ‘reluctance’ regarding vaccination. Following our analysis, we interpreted these descriptions as different ways of how VH may be affected, experienced or expressed at an individual level, representing a layer surrounding the central element of VH. We therefore interpret cognitions and affect to go hand-in-hand, but not to be at the core of hesitancy. Moreover, we conclude that cognitions or affect are insufficiently distinctive to define VH.
This interpretation does not mean that the identified cognitions or affect are irrelevant to VH. On the contrary, they may prove crucial in shaping VH. However, to arrive at a clear definition of VH, cognitions and affects should be treated as clearly defined entities as well. Only by unravelling and distinguishing them can the exact nature of their relationship with VH be clarified in further research.
In the conceptualization decision making, VH was described as being ‘undecided’, ‘indecisive’, ‘in consideration’ or ‘not yet making a vaccine decision’. All these descriptions include an element of indecision, and this provides a unique and distinctive feature for VH. Additionally, we found that this conceptualization is predominantly discussed in studies in the public health field. This is rather logical, as one would expect this field of research to take a more pragmatic approach, examining the presence of VH at a stage where people have been offered a vaccine or to anticipate public sentiments around willingness to accept a vaccine when it is offered. This probably triggers a decision-making process where VH can emerge and manifest. On the basis of these findings, we argue that VH is a psychological state of being undecided, indecisive or not yet making a decision regarding vaccination.
The study selection was conducted independently by different members of our research team. However, one possible limitation is that we did not attempt to exclude studies of lower quality, as we wanted to maintain a robust selection of studies to enable a broad overview of the relevant literature. Our MMAT assessment, however, indicates that the majority of the studies are of medium quality. A second limitation is that a considerable number of the included conceptual studies (17 of the 36) 14 , 18 , 20 , 21 , 22 , 23 , 25 , 26 , 29 , 35 , 38 , 39 , 40 , 41 , 42 , 43 , 44 quoted the VH definition introduced by the SAGE working group, which may have led to an amplification of the SAGE definition. This may indicate that this definition is well recognized, but potentially overshadows less recognized conceptual definitions of VH. We chose to include all quoted definitions and found that many studies used more than one. We did not look further into conflicting definitions within the articles, but doing so could yield interesting insights.
In conclusion, we propose a definition of VH as a psychological state of indecisiveness that people may experience when making a decision regarding vaccination. We acknowledge that experiencing concerns, doubts or reluctance regarding vaccination may play a vital role in shaping VH. However, we argue that these factors have the highest potential to advance scientific knowledge when treated as relevant constructs integral to shaping VH, rather than treating them as synonymous to VH. Operationalizing VH by measuring or distinguishing subpopulations should ideally be directed at this state of indecision. To avoid confusion, it is important to separate VH from vaccination behaviour, which is already a well-defined concept. This proposal of a renewed definition of a concept that has been used for a decade could be perceived as ‘putting old wine in new bottles’. However, we feel that due to the large amount of highly varied literature, and given the importance of VH research in predicting, explaining and influencing immunization behaviour, it is necessary to take a snapshot of the status quo. The conclusion of this review is that VH is, for now, an impracticable concept, due to the confusing use of multiple, varied operationalizations. To aid further research, the VH concept must be clearly conceptualized and adapted from its broad and inclusive form to a pragmatic and refined alternative. Working on such an alternative, the field should first reach consensus on the definition and then measure VH accordingly. This approach allows for a much-needed comparison between studies to improve our understanding of VH determinants, correlates and consequences on an individual and societal level. Our way forward is to simplify and clarify the operationalization of VH by returning to its root core of indecisiveness.
This systematic review was registered on 11 November 2020 in the PROSPERO database (CRD42020211046). The record and study protocol are available at https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=211046 .
Relevant publications were searched using the PubMed, Embase and PsycINFO databases to ensure coverage of all relevant research areas in the medical, public health and social science fields. The CINAHL database was also considered, but a pilot search revealed that its unique contributions were limited.
An experienced research librarian used the following keywords to develop a search strategy (Supplementary Methods ): ‘vaccination’, ‘immunization’, ‘vaccination refusal’, ‘vaccination avoidance’, ‘vaccination hesitation’, ‘vaccine hesitancy’, ‘vaccine uptake’, ‘vaccination behaviour’, ‘vaccination attitude’, ‘vaccine confidence’, ‘vaccine acceptance’ and ‘vaccine barriers’. The limitations included a publication date of between 2010 and the date of the search (14 January 2022). Conference abstracts were excluded from the search of the Embase database.
Eligibility criteria
The included studies were all published in peer-reviewed journals and written in English. All study types were eligible, except editorials and commentaries, as we sought to include original studies. Studies on animal vaccines were excluded.
The purpose of this review was to clarify the VH concept by analysing how it is operationalized. We recognized operationalizations at two main levels: conceptual and empirical. This resulted in three main groups: (1) studies describing or defining the VH concept and studies applying the concept by (2) identifying VH subpopulations and (3) measuring VH in individuals. This approach allowed comparison between conceptual and empirical operationalizations of VH.
Study selection
In the first selection round, two members of the research team used RAYYAN software to independently assess the titles and abstracts. Studies were selected when the title or abstract contained the term ‘vaccine hesitancy’. Studies were also selected if the title or abstract indicated that the full text contained further information on VH conceptualization, subpopulations or measurements. Papers without an abstract were selected for full-text screening. After double-screening, the results were de-blinded to allow the researchers to discuss their conflicting judgements until consensus was reached.
In the second selection round, the full texts were screened. The first 30% of studies were double-screened to establish a uniform method. Studies were screened on whether they met the criteria for one or more of the three categories (conceptualization, subpopulations and measurements). The category of ‘conceptualization’ included studies that describe, discuss or explore the VH concept or propose a novel VH measurement instrument. Studies falling into only the second category (subpopulations) were excluded if they merely distinguished between hesitant and non-hesitant groups, since a dichotomous grouping does not contribute to understanding of VH. The references from the included full-text articles were screened to find additional studies matching the selection criteria.
We deviated posthoc from our preregistered study protocol by adjusting the study selection criteria as follows. Initially, we also included studies containing the term ‘vaccine confidence’ (that is, with no mention or operationalization of vaccine hesitancy) as indicated in our study protocol. During the process, we realized that this deviated from our primary aim to clarify the VH concept by differentiating its related concepts. Therefore, we adapted the protocol and excluded 16 studies that were exclusively on vaccine confidence from our analysis
Data collection
The study characteristics were extracted from each of the full-text articles. Data were extracted by one researcher and verified by a second member of the research team. The variables included the first author, year of publication, research field of the first author, type of study, type of participants, number of participants, type of vaccination and country in which the study was conducted (with corresponding economic status) 447 . For the studies that do not include data collection, the country of origin was determined using the affiliation of the first author.
From the studies on VH conceptualization, text excerpts that define or describe VH or describe the relationship of VH to other concepts were extracted. These excerpts were further analysed in the qualitative analysis. From studies that describe different VH subpopulations, information about the categorization of these various subgroups was extracted, including the rationale for the distinguished subpopulations. From studies that describe VH measurements, the instrument(s) and criteria used to define VH were extracted.
Synthesis of results
The text excerpts extracted from the studies conceptualizing VH were thematically coded using ATLAS.ti software. Three research team members developed a coding book of themes and subthemes after independent coding of 30% of the studies. Thereafter, one researcher continued the coding process for the remaining studies. Any emerging new codes were discussed with the other research team members. The results were analysed qualitatively, and the predominant themes were identified by the three team members. When possible, results were grouped by research field and vaccine type to allow for comparison.
The data extracted from the studies describing VH subpopulations were summarized in a table and grouped according to the common themes identified. The data extracted from the studies describing a VH measurement were summarized in a table and grouped according to the instrument or method used. Where multiple measurement instruments are used in one study, the tool used to determine hesitancy was selected as the main instrument.
Quality assessment
The quality of each study was assessed using the MMAT 448 . This tool contains appraisal guidelines for different study types, covering the majority of the included studies. An overall score was calculated (1–5) on the basis of additional communication about the MMAT 2018 version, with higher scores indicating higher quality levels 449 . The first 20% of studies were assessed independently by two members of the research team to ensure consistency. Thereafter, one member of the research team continued the assessment.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
All data generated or analysed during this study are included in this article and its Supplementary Information . This systematic review is registered in PROSPERO (CRD42020211046).
Ten Threats to Global Health in 2019 (WHO, 2019); https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019
Patel, M. K. et al. Progress toward regional measles elimination - worldwide, 2000–2019. MMWR Morb. Mortal. Wkly Rep. 69 , 1700–1705 (2020).
Article Google Scholar
Suijkerbuijk, A. W. et al. Economic costs of measles outbreak in the Netherlands, 2013–2014. Emerg. Infect. Dis. 21 , 2067–2069 (2015).
Ghebrehewet, S. et al. The economic cost of measles: healthcare, public health and societal costs of the 2012–13 outbreak in Merseyside, UK. Vaccine 34 , 1823–1831 (2016).
Chovatiya, R. & Silverberg, J. I. Inpatient morbidity and mortality of measles in the United States. PLoS ONE 15 , e0231329 (2020).
Article CAS Google Scholar
Feemster, K. A. & Szipszky, C. Resurgence of measles in the United States: how did we get here? Curr. Opin. Pediatr. 32 , 139–144 (2020).
Rechel, B., Richardson, E. & McKee, M. The Organization and Delivery of Vaccination Services in the European Union (The European Observatory on Health Systems and Policies, 2018). https://www.euro.who.int/__data/assets/pdf_file/0008/386684/vaccination-report-eng.pdf
Wilder-Smith, A. B. & Qureshi, K. Resurgence of measles in Europe: a systematic review on parental attitudes and beliefs of measles vaccine. J. Epidemiol. Glob. Health 10 , 46–58 (2020).
Bangura, J. B., Xiao, S., Qiu, D., Ouyang, F. & Chen, L. Barriers to childhood immunization in sub-Saharan Africa: a systematic review. BMC Public Health 20 , 1108 (2020).
Sweileh, W. M. Bibliometric analysis of global scientific literature on vaccine hesitancy in peer-reviewed journals (1990–2019). BMC Public Health 20 , 1252 (2020).
Frederiksen, L. S. F., Zhang, Y., Foged, C. & Thakur, A. The long road toward COVID-19 herd immunity: vaccine platform technologies and mass immunization strategies. Front. Immunol. 11 , 1817 (2020).
Randolph, H. E. & Barreiro, L. B. Herd immunity: understanding COVID-19. Immunity 52 , 737–741 (2020).
Dror, A. A. et al. Vaccine hesitancy: the next challenge in the fight against COVID-19. Eur. J. Epidemiol. 35 , 775–779 (2020).
MacDonald, N. E. Vaccine hesitancy: definition, scope and determinants. Vaccine 33 , 4161–4164 (2015).
Podsakoff, P. M., MacKenzie, S. B. & Podsakoff, N. P. Recommendations for creating better concept definitions in the organizational, behavioral, and social sciences. Organ. Res. Methods 19 , 159–203 (2016).
Shapiro, G. K. et al. A critical review of measures of childhood vaccine confidence. Curr. Opin. Immunol. 71 , 34–45 (2021).
Brewer, N. T., Chapman, G. B., Rothman, A. J., Leask, J. & Kempe, A. Increasing vaccination: putting psychological science into action. Psychol. Sci. Public Interest 18 , 149–207 (2017).
Bedford, H. et al. Vaccine hesitancy, refusal and access barriers: the need for clarity in terminology. Vaccine 36 , 6556–6558 (2018).
Orenstein, W. et al. Assessing the state of vaccine confidence in the United States: recommendations from the national vaccine advisory committee. Public Health Rep. 130 , 573–595 (2015).
Schmid, P., Rauber, D., Betsch, C., Lidolt, G. & Denker, M. L. Barriers of influenza vaccination intention and behavior - a systematic review of influenza vaccine hesitancy, 2005–2016. PLoS ONE 12 , e0170550 (2017).
Dubé, E. et al. “Nature does things well, why should we interfere?”: vaccine hesitancy among mothers. Qual. Health Res. 26 , 411–425 (2016).
Betsch, C. et al. Beyond confidence: development of a measure assessing the 5C psychological antecedents of vaccination. PLoS ONE 13 , e0208601 (2018).
Peretti-Watel, P., Larson, H. J., Ward, J. K., Schulz, W. S. & Verger, P. Vaccine hesitancy: clarifying a theoretical framework for an ambiguous notion. PLoS Curr. https://doi.org/10.1371/currents.outbreaks.6844c80ff9f5b273f34c91f71b7fc289 (2015).
Page, M. J. et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Brit. Med. J. 372 , n71 (2021).
Bertoncello, C. et al. Socioeconomic determinants in vaccine hesitancy and vaccine refusal in Italy. Vaccines 8 , 276 (2020).
Deml, M. J. et al. ‘Problem patients and physicians’ failures’: what it means for doctors to counsel vaccine hesitant patients in Switzerland. Soc. Sci. Med. 255 , 112946 (2020).
Dubé, E. et al. Vaccine hesitancy: an overview. Hum. Vaccin. Immunother. 9 , 1763–1773 (2013).
Dubé, E., Gagnon, D., Nickels, E., Jeram, S. & Schuster, M. Mapping vaccine hesitancy–country-specific characteristics of a global phenomenon. Vaccine 32 , 6649–6654 (2014).
Dubé, E. et al. Understanding vaccine hesitancy in Canada: results of a consultation study by the Canadian Immunization Research Network. PLoS ONE 11 , e0156118 (2016).
Gowda, C. & Dempsey, A. F. The rise (and fall?) of parental vaccine hesitancy. Hum. Vaccin. Immunother. 9 , 1755–1762 (2013).
Kestenbaum, L. A. & Feemster, K. A. Identifying and addressing vaccine hesitancy. Pediatr. Ann. 44 , e71–e75 (2015).
Larson, H. J. Negotiating vaccine acceptance in an era of reluctance. Hum. Vaccin. Immunother. 9 , 1779–1781 (2013).
Oduwole, E. O., Pienaar, E. D., Mahomed, H. & Wiysonge, C. S. Current tools available for investigating vaccine hesitancy: a scoping review protocol. BMJ Open 9 , e033245 (2019).
Opel, D. J. et al. Development of a survey to identify vaccine-hesitant parents: the parent attitudes about childhood vaccines survey. Hum. Vaccin. 7 , 419–425 (2011).
Smith, M. J. Promoting vaccine confidence. Infect. Dis. Clin. North Am. 29 , 759–769 (2015).
Dubé, È., Ward, J. K., Verger, P. & MacDonald, N. E. Vaccine hesitancy, acceptance, and anti-vaccination: trends and future prospects for public health. Annu Rev. Public Health 42 , 175–191 (2021).
Popper-Giveon, A. & Keshet, Y. Non-Vaccination Stage Model (NVST): the decision-making process among Israeli ultra-orthodox Jewish parents. Health https://doi.org/10.1177/1363459320988884 (2021).
Kumar, D., Chandra, R., Mathur, M., Samdariya, S. & Kapoor, N. Vaccine hesitancy: understanding better to address better. Isr. J. Health Policy Res . 5 , 2 (2016).
McIntosh, E. D., Janda, J., Ehrich, J. H., Pettoello-Mantovani, M. & Somekh, E. Vaccine hesitancy and refusal. J. Pediatr. 175 , 248–249.e1 (2016).
Díaz Crescitelli, M. E. et al. A meta-synthesis study of the key elements involved in childhood vaccine hesitancy. Public Health 180 , 38–45 (2020).
Larson, H. J. et al. Measuring vaccine hesitancy: the development of a survey tool. Vaccine 33 , 4165–4175 (2015).
Howard, M. C. A more comprehensive measure of vaccine hesitancy: creation of the Multidimensional Vaccine Hesitancy Scale (MVHS). J. Health Psychol . https://doi.org/10.1177/13591053211042062 (2021).
Turner, P. J., Larson, H., Dubé, È. & Fisher, A. Vaccine hesitancy: drivers and how the allergy community can help. J. Allergy Clin. Immunol. Pract. 9 , 3568–3574 (2021).
Freeman, D. et al. COVID-19 vaccine hesitancy in the UK: the Oxford coronavirus explanations, attitudes, and narratives survey (Oceans) II. Psychol. Med . https://doi.org/10.1017/s0033291720005188 (2020).
Su, Z. et al. A race for a better understanding of COVID-19 vaccine non-adopters. Brain Behav. Immun. Health 9 , 100159 (2020).
Sarathchandra, D., Navin, M. C., Largent, M. A. & McCright, A. M. A survey instrument for measuring vaccine acceptance. Prev. Med. 109 , 1–7 (2018).
Leask, J. et al. Communicating with parents about vaccination: a framework for health professionals. BMC Pediatr. 12 , 154 (2012).
Jankovic, S. Childhood vaccination in the twenty-first century: parental concerns and challenges for physicians. Arh. Farm. 69 , 452–468 (2019).
Hagood, E. A. & Mintzer Herlihy, S. Addressing heterogeneous parental concerns about vaccination with a multiple-source model: a parent and educator perspective. Hum. Vaccin. Immunother. 9 , 1790–1794 (2013).
Blaisdell, L. L., Gutheil, C., Hootsmans, N. A. & Han, P. K. Unknown risks: parental hesitation about vaccination. Med. Decis. Mak. 36 , 479–489 (2016).
Amin, A. B. et al. Association of moral values with vaccine hesitancy. Nat. Hum. Behav. 1 , 873–880 (2017).
Kotta, I., Kalcza-Janosi, K., Szabo, K. & Marschalko, E. E. Development and validation of the multidimensional COVID-19 vaccine hesitancy scale. Hum. Vaccin. Immunother. 18 , 1–10 (2021).
Sjögren, E., Ask, L. S., Örtqvist, Å. & Asp, M. Parental conceptions of the rotavirus vaccine during implementation in Stockholm: a phenomenographic study. J. Child Health Care 21 , 476–487 (2017).
Frew, P. M. et al. Development of a US trust measure to assess and monitor parental confidence in the vaccine system. Vaccine 37 , 325–332 (2019).
Smith, J. C., Appleton, M. & MacDonald, N. E. Building confidence in vaccines. Adv. Exp. Med. Biol. 764 , 81–98 (2013).
Larson, H. J., Schulz, W. S., Tucker, J. D. & Smith, D. M. Measuring vaccine confidence: introducing a global vaccine confidence index. PLoS Curr. https://doi.org/10.1371/currents.outbreaks.ce0f6177bc97332602a8e3fe7d7f7cc4 (2015).
Ramanadhan, S., Galarce, E., Xuan, Z., Alexander-Molloy, J. & Viswanath, K. Addressing the vaccine hesitancy continuum: an audience segmentation analysis of american adults who did not receive the 2009 H1N1 vaccine. Vaccines 3 , 556–578 (2015).
Rossen, I., Hurlstone, M. J., Dunlop, P. D. & Lawrence, C. Accepters, fence sitters, or rejecters: moral profiles of vaccination attitudes. Soc. Sci. Med. 224 , 23–27 (2019).
Rozbroj, T., Lyons, A. & Lucke, J. Vaccine-hesitant and vaccine-refusing parents’ reflections on the way parenthood changed their attitudes to vaccination. J. Community Health 45 , 63–72 (2020).
Schwartz, J. L. & Caplan, A. L. Vaccination refusal: ethics, individual rights, and the common good. Prim. Care 38 , 717–728 (2011).
Shay, L. A. et al. Parent-provider communication of HPV vaccine hesitancy. Pediatrics 141 , e20172312 (2018).
Oladejo, O. et al. Comparative analysis of the Parent Attitudes about Childhood Vaccines (PACV) short scale and the five categories of vaccine acceptance identified by Gust et al. Vaccine 34 , 4964–4968 (2016).
Bouchez, M. et al. Physicians’ decision processes about the HPV vaccine: a qualitative study. Vaccine 39 , 521–528 (2021).
Elwy, A. R. et al. Vaccine hesitancy as an opportunity for engagement: a rapid qualitative study of patients and employees in the U.S. Veterans Affairs healthcare system. Vaccine 9 , 100116 (2021).
CAS Google Scholar
Paris, C. et al. COVID-19 vaccine hesitancy among healthcare workers. Infect. Dis. Now 51 , 484–487 (2021).
Tram, K. H. et al. Deliberation, dissent, and distrust: understanding distinct drivers of COVID-19 vaccine hesitancy in the United States. Clin. Infect. Dis. https://doi.org/10.1093/cid/ciab633 (2021).
Vulpe, S. N. & Rughiniş, C. Social amplification of risk and “probable vaccine damage”: a typology of vaccination beliefs in 28 European countries. Vaccine 39 , 1508–1515 (2021).
Wang, M. W. et al. COVID-19 vaccination acceptance among healthcare workers and non-healthcare workers in china: a survey. Front. Public Health 9 , 709056 (2021).
Shapiro, G. K. et al. Using an integrated conceptual framework to investigate parents’ HPV vaccine decision for their daughters and sons. Prev. Med. 116 , 203–210 (2018).
Tatar, O., Shapiro, G. K., Perez, S., Wade, K. & Rosberger, Z. Using the precaution adoption process model to clarify human papillomavirus vaccine hesitancy in Canadian parents of girls and parents of boys. Hum. Vaccin. Immunother. 15 , 1803–1814 (2019).
Dubov, A. et al. Predictors of COVID-19 vaccine acceptance and hesitancy among healthcare workers in Southern California: not just “anti” vs. “pro” vaccine. Vaccines 9 , 1428 (2021).
Brunelli, L. et al. Parental trust and beliefs after the discovery of a six-year-long failure to vaccinate. Hum. Vaccin. Immunother. 17 , 583–587 (2020).
Armiento, R. et al. Impact of Australian mandatory ‘No Jab, No Pay’ and ‘No Jab, No Play’ immunisation policies on immunisation services, parental attitudes to vaccination and vaccine uptake, in a tertiary paediatric hospital, the Royal Children’s Hospital, Melbourne. Vaccine 38 , 5231–5240 (2020).
Berry, N. J. et al. Sharing knowledge about immunisation (SKAI): an exploration of parents’ communication needs to inform development of a clinical communication support intervention. Vaccine 36 , 6480–6490 (2018).
Bocquier, A. et al. Social differentiation of vaccine hesitancy among French parents and the mediating role of trust and commitment to health: a nationwide cross-sectional study. Vaccine 36 , 7666–7673 (2018).
Chang, K. & Lee, S. Y. Why do some Korean parents hesitate to vaccinate their children? Epidemiol. Health 41 , e2019031 (2019).
Danchin, M. H. et al. Vaccine decision-making begins in pregnancy: correlation between vaccine concerns, intentions and maternal vaccination with subsequent childhood vaccine uptake. Vaccine 36 , 6473–6479 (2018).
Forbes, T. A., McMinn, A., Crawford, N., Leask, J. & Danchin, M. Vaccination uptake by vaccine-hesitant parents attending a specialist immunization clinic in Australia. Hum. Vaccin. Immunother. 11 , 2895–2903 (2015).
Glanternik, J. R. et al. Evaluation of a vaccine-communication tool for physicians. J. Pediatr. 224 , 72–78.e1 (2020).
McDonald, P. et al. Exploring California’s new law eliminating personal belief exemptions to childhood vaccines and vaccine decision-making among homeschooling mothers in California. Vaccine 37 , 742–750 (2019).
Noyman-Veksler, G., Greenberg, D., Grotto, I. & Shahar, G. Parents’ malevolent personification of mass vaccination solidifies vaccine hesitancy. J. Health Psychol . https://doi.org/10.1177/1359105320903475 (2020).
Duong, M. C., Nguyen, H. T. & Duong, M. Evaluating COVID-19 vaccine hesitancy: a qualitative study from Vietnam. Diabetes Metab. Syndr. 16 , 102363 (2022).
Gatto, N. M. et al. Correlates of COVID-19 vaccine acceptance, hesitancy and refusal among employees of a safety net California County health system with an early and aggressive vaccination program: results from a cross-sectional survey. Vaccines 9 , 1152 (2021).
Moore, J. X. et al. Correlates of COVID-19 vaccine hesitancy among a community sample of African Americans living in the southern United States. Vaccines 9 , 879 (2021).
Murphy, J. et al. Psychological characteristics associated with COVID-19 vaccine hesitancy and resistance in Ireland and the United Kingdom. Nat. Commun. 12 , 29 (2021).
Musa, S. et al. A qualitative interview study with parents to identify barriers and drivers to childhood vaccination and inform public health interventions. Hum. Vaccin. Immunother. 17 , 3023–3033 (2021).
Steffens, M. S., Bullivant, B., Bolsewicz, K. T., King, C. & Beard, F. “to protect myself, my friends, family, workmates and patients… and to play my part”: COVID-19 vaccination perceptions among health and aged care workers in New South Wales, Australia. Int. J. Environ. Res. Public Health 18 , 8954 (2021).
Tavolacci, M. P., Dechelotte, P. & Ladner, J. COVID-19 vaccine acceptance, hesitancy, and resistancy among university students in France. Vaccines 9 , 654 (2021).
Buttenheim, A. M. et al. Vaccine exemption requirements and parental vaccine attitudes: an online experiment. Vaccine 38 , 2620–2625 (2020).
Corben, P. & Leask, J. Vaccination hesitancy in the antenatal period: a cross-sectional survey. BMC Public Health 18 , 566 (2018).
Gagneur, A. et al. Promoting vaccination in maternity wards ─ motivational interview technique reduces hesitancy and enhances intention to vaccinate, results from a multicentre non-controlled pre- and post-intervention RCT-nested study, Quebec, March 2014 to February 2015. Eur. Surveill. 24 , 1800641 (2019).
Lau, L. H. W., Lee, S. S. & Wong, N. S. The continuum of influenza vaccine hesitancy among nursing professionals in Hong Kong. Vaccine 38 , 6785–6793 (2020).
Nekrasova, E. et al. Vaccine hesitancy and influenza beliefs among parents of children requiring a second dose of influenza vaccine in a season: an American Academy of Pediatrics (AAP) Pediatric Research in Office Settings (PROS) study. Hum. Vaccin. Immunother. 16 , 1070–1077 (2020).
Verger, P. et al. Prevalence and correlates of vaccine hesitancy among general practitioners: a cross-sectional telephone survey in France, April to July 2014. Eur. Surveill. 21 , 30406 (2016).
Bianco, A. et al. Parental COVID-19 vaccine hesitancy: a cross-sectional survey in Italy. Expert Rev. Vaccines 21 , 541–547 (2022).
Edwards, B., Biddle, N., Gray, M. & Sollis, K. COVID-19 vaccine hesitancy and resistance: correlates in a nationally representative longitudinal survey of the Australian population. PLoS ONE 16 , e0248892 (2021).
Hsieh, Y. L., Rak, S., SteelFisher, G. K. & Bauhoff, S. Effect of the suspension of the J&J COVID-19 vaccine on vaccine hesitancy in the United States. Vaccine 40 , 424–427 (2022).
Umakanthan, S., Patil, S., Subramaniam, N. & Sharma, R. COVID-19 vaccine hesitancy and resistance in India explored through a population-based longitudinal survey. Vaccines 9 , 1064 (2021).
Zheng, W. et al. COVID-19 vaccine uptake and hesitancy among HIV-infected men who have sex with men in mainland China: a cross-sectional survey. Hum. Vaccin. Immunother. 17 , 4971–4981 (2021).
Baniak, L. M., Luyster, F. S., Raible, C. A., McCray, E. E. & Strollo, P. J. COVID-19 vaccine hesitancy and uptake among nursing staff during an active vaccine rollout. Vaccines 9 , 858 (2021).
Bou Hamdan, M., Singh, S., Polavarapu, M., Jordan, T. R. & Melhem, N. M. COVID-19 vaccine hesitancy among university students in Lebanon. Epidemiol. Infect. 149 , e242 (2021).
Castellano-Tejedor, C., Torres-Serrano, M. & Cencerrado, A. Unveiling Associations of COVID-19 vaccine acceptance, hesitancy, and resistance: a cross-sectional community-based adult survey. Int. J. Environ. Res. Public Health 18 , 12348 (2021).
Freeman, D. et al. Effects of different types of written vaccination information on COVID-19 vaccine hesitancy in the UK (OCEANS-III): a single-blind, parallel-group, randomised controlled trial. Lancet Public Health 6 , e416–e427 (2021).
Janssen, C. et al. Hesitancy towards COVID-19 vaccination among healthcare workers: a multi-centric survey in France. Vaccines 9 , 547 (2021).
Khaled, S. M. et al. Prevalence and potential determinants of COVID-19 vaccine hesitancy and resistance in Qatar: results from a nationally representative survey of Qatari nationals and migrants between December 2020 and January 2021. Vaccines 9 , 471 (2021).
Montagni, I. et al. Acceptance of a Covid-19 vaccine is associated with ability to detect fake news and health literacy. J. Public Health https://doi.org/10.1093/pubmed/fdab028 (2021).
Qunaibi, E., Basheti, I., Soudy, M. & Sultan, I. Hesitancy of Arab healthcare workers towards COVID-19 vaccination: a large-scale multinational study. Vaccines 9 , 446 (2021).
Reno, C. et al. Enhancing COVID-19 vaccines acceptance: results from a survey on vaccine hesitancy in northern Italy. Vaccines 9 , 378 (2021).
Rodríguez-Blanco, N. et al. Willingness to be vaccinated against COVID-19 in Spain before the start of vaccination: a cross-sectional study. Int. J. Environ. Res. Public Health 18 , 5272 (2021).
Leung, C. L. K. et al. Profiling vaccine believers and skeptics in nurses: a latent profile analysis. Int. J. Nurs. Stud. 126 , 104142 (2021).
Salerno, L., Craxì, L., Amodio, E. & Lo Coco, G. Factors affecting hesitancy to mRNA and viral vector COVID-19 vaccines among college students in Italy. Vaccines 9 , 927 (2021).
Zona, S. et al. Anti-COVID vaccination for adolescents: a survey on determinants of vaccine parental hesitancy. Vaccines 9 , 1309 (2021).
Alabbad, A. A., Alsaad, A. K., Al Shaalan, M. A., Alola, S. & Albanyan, E. A. Prevalence of influenza vaccine hesitancy at a tertiary care hospital in Riyadh, Saudi Arabia. J. Infect. Public Health 11 , 491–499 (2018).
Barello, S., Nania, T., Dellafiore, F., Graffigna, G. & Caruso, R. ‘Vaccine hesitancy’ among university students in Italy during the COVID-19 pandemic. Eur. J. Epidemiol. 35 , 781–783 (2020).
Bogart, L. M. et al. COVID-19 related medical mistrust, health impacts, and potential vaccine hesitancy among Black Americans living with HIV. J. Acquir. Immune Defic. Syndr. 86 , 200–207 (2020).
Brown, A. L. et al. Vaccine confidence and hesitancy in Brazil. Cad. Saude Publica 34 , e00011618 (2018).
Byström, E., Lindstrand, A., Bergström, J., Riesbeck, K. & Roth, A. Confidence in the National Immunization Program among parents in Sweden 2016 – a cross-sectional survey. Vaccine 38 , 3909–3917 (2020).
Costantino, C. et al. Determinants of vaccine hesitancy and effectiveness of vaccination counseling interventions among a sample of the general population in Palermo, Italy. Hum. Vaccin. Immunother. 16 , 2415–2421 (2020).
Deas, J., Bean, S. J., Sokolovska, I. & Fautin, C. Childhood vaccine attitudes and information sources among Oregon parents and guardians. Health Promot. Pract. 20 , 529–538 (2019).
Dempsey, A. F., Pyrzanowski, J., Campagna, E. J., Lockhart, S. & O’Leary, S. T. Parent report of provider HPV vaccine communication strategies used during a randomized, controlled trial of a provider communication intervention. Vaccine 37 , 1307–1312 (2019).
Detoc, M. et al. Intention to participate in a COVID-19 vaccine clinical trial and to get vaccinated against COVID-19 in France during the pandemic. Vaccine 38 , 7002–7006 (2020).
Du, F. et al. The determinants of vaccine hesitancy in China: a cross-sectional study following the Changchun Changsheng vaccine incident. Vaccine 38 , 7464–7471 (2020).
Dubé, E., Gagnon, D., Zhou, Z. & Deceuninck, G. Parental vaccine hesitancy in Quebec (Canada). PLoS Curr . https://doi.org/10.1371/currents.outbreaks.9e239605f4d320c6ad27ce2aea5aaad2 (2016).
Fisher, K. A. et al. Attitudes toward a potential SARS-CoV-2 vaccine: a survey of U.S. adults. Ann. Intern. Med. 173 , 964–973 (2020).
Gesser-Edelsburg, A., Walter, N., Shir-Raz, Y., Sassoni Bar-Lev, O. & Rosenblat, S. The behind-the-scenes activity of parental decision-making discourse regarding childhood vaccination. Am. J. Infect. Control 45 , 267–271 (2017).
Gowda, C., Schaffer, S. E., Kopec, K., Markel, A. & Dempsey, A. F. A pilot study on the effects of individually tailored education for MMR vaccine-hesitant parents on MMR vaccination intention. Hum. Vaccin. Immunother. 9 , 437–445 (2013).
Gowda, C., Schaffer, S. E., Kopec, K., Markel, A. & Dempsey, A. F. Does the relative importance of MMR vaccine concerns differ by degree of parental vaccine hesitancy?: an exploratory study. Hum. Vaccin. Immunother. 9 , 430–436 (2013).
Hadjipanayis, A. et al. Vaccine confidence among parents: large scale study in eighteen European countries. Vaccine 38 , 1505–1512 (2020).
Hirth, J. M., Fuchs, E. L., Chang, M., Fernandez, M. E. & Berenson, A. B. Variations in reason for intention not to vaccinate across time, region, and by race/ethnicity, NIS-Teen (2008–2016). Vaccine 37 , 595–601 (2019).
Karlsson, L. C. et al. The association between vaccination confidence, vaccination behavior, and willingness to recommend vaccines among Finnish healthcare workers. PLoS ONE 14 , e0224330 (2019).
Khodadadi, A. B., Redden, D. T. & Scarinci, I. C. HPV vaccination hesitancy among latina immigrant mothers despite physician recommendation. Ethn. Dis. 30 , 661–670 (2020).
Melot, B. et al. Knowledge, attitudes and practices about vaccination in Trentino, Italy in 2019. Hum. Vaccin. Immunother. 17 , 259–268 (2020).
Mereu, N. et al. Vaccination attitude and communication in early settings: an exploratory study. Vaccines 8 , 701 (2020).
Opel, D. J. et al. Characterizing providers’ immunization communication practices during health supervision visits with vaccine-hesitant parents: a pilot study. Vaccine 30 , 1269–1275 (2012).
Özceylan, G., Toprak, D. & Esen, E. S. Vaccine rejection and hesitation in Turkey. Hum. Vaccin. Immunother. 16 , 1034–1039 (2020).
Pahud, B. et al. A randomized controlled trial of an online immunization curriculum. Vaccine 38 , 7299–7307 (2020).
Quinn, S. C. et al. Breaking down the monolith: understanding flu vaccine uptake among African Americans. SSM Popul. Health 4 , 25–36 (2018).
Quinn, S. C. et al. Exploring racial influences on flu vaccine attitudes and behavior: results of a national survey of White and African American adults. Vaccine 35 , 1167–1174 (2017).
Quinn, S. C., Jamison, A. M., An, J., Hancock, G. R. & Freimuth, V. S. Measuring vaccine hesitancy, confidence, trust and flu vaccine uptake: results of a national survey of White and African American adults. Vaccine 37 , 1168–1173 (2019).
Repalust, A., Šević, S., Rihtar, S. & Štulhofer, A. Childhood vaccine refusal and hesitancy intentions in Croatia: insights from a population-based study. Psychol. Health Med. 22 , 1045–1055 (2017).
Rey, D. et al. Vaccine hesitancy in the French population in 2016, and its association with vaccine uptake and perceived vaccine risk-benefit balance. Eur. Surveill. 23 , 17–00816 (2018).
Santibanez, T. A. et al. Parental vaccine hesitancy and childhood influenza vaccination. Pediatrics 146 , e2020007609 (2020).
Schollin Ask, L. et al. Receiving early information and trusting Swedish child health centre nurses increased parents’ willingness to vaccinate against rotavirus infections. Acta Paediatr. 106 , 1309–1316 (2017).
Suryadevara, M., Handel, A., Bonville, C. A., Cibula, D. A. & Domachowske, J. B. Pediatric provider vaccine hesitancy: an under-recognized obstacle to immunizing children. Vaccine 33 , 6629–6634 (2015).
Taylor, S. et al. A proactive approach for managing COVID-19: the importance of understanding the motivational roots of vaccination hesitancy for SARS-CoV2. Front. Psychol. 11 , 575950 (2020).
Tran, B. X. et al. Media representation of vaccine side effects and its impact on utilization of vaccination services in Vietnam. Patient Prefer. Adherence 12 , 1717–1728 (2018).
Wilson, R. et al. Vaccine hesitancy and self-vaccination behaviors among nurses in southeastern France. Vaccine 38 , 1144–1151 (2020).
Yu, W. et al. Two media-reported vaccine events in China from 2013 to 2016: impact on confidence and vaccine utilization. Vaccine 38 , 5541–5547 (2020).
Abdel-Qader, D. H. et al. Pharmacists-physicians collaborative intervention to reduce vaccine hesitancy and resistance: a randomized controlled trial. Vaccine X 10 , 100135 (2022).
Abedin, M. et al. Willingness to vaccinate against COVID-19 among Bangladeshi adults: understanding the strategies to optimize vaccination coverage. PLoS ONE 16 , e0250495 (2021).
Abou Leila, R., Salamah, M. & El-Nigoumi, S. Reducing COVID-19 vaccine hesitancy by implementing organizational intervention in a primary care setting in Bahrain. Cureus 13 , e19282 (2021).
Google Scholar
Aguilar Ticona, J. P. et al. Willingness to get the COVID-19 vaccine among residents of slum settlements. Vaccines 9 , 951 (2021).
Ahmad, K. K. Coronavirus disease 2019 vaccine hesitancy in the Kurdistan region: a cross-sectional national survey. Arch. Razi Inst. 76 , 751–759 (2021).
Al-Ayyadhi, N., Ramadan, M. M., Al-Tayar, E., Al-Mathkouri, R. & Al-Awadhi, S. Determinants of hesitancy towards COVID-19 vaccines in State of Kuwait: an exploratory internet-based survey. Risk Manage. Healthc. Policy 14 , 4967–4981 (2021).
Alfieri, N. L. et al. Parental COVID-19 vaccine hesitancy for children: vulnerability in an urban hotspot. BMC Public Health 21 , 1662 (2021).
Alibrahim, J. & Awad, A. COVID-19 vaccine hesitancy among the public in Kuwait: a cross-sectional survey. Int. J. Environ. Res. Public Health 18 , 8836 (2021).
Allington, D., McAndrew, S., Moxham-Hall, V. & Duffy, B. Coronavirus conspiracy suspicions, general vaccine attitudes, trust and coronavirus information source as predictors of vaccine hesitancy among UK residents during the COVID-19 pandemic. Psychol. Med . https://doi.org/10.1017/s0033291721001434 (2021).
Al-Sanafi, M. & Sallam, M. Psychological determinants of COVID-19 vaccine acceptance among healthcare workers in Kuwait: a cross-sectional study using the 5C and vaccine conspiracy beliefs scales. Vaccines 9 , 701 (2021).
Alshahrani, S. M. et al. Acceptability of COVID-19 vaccination in Saudi Arabia: a cross-sectional study using a web-based survey. Hum. Vaccin. Immunother. 17 , 3338–3347 (2021).
Al-Wutayd, O., Khalil, R. & Rajar, A. B. Sociodemographic and behavioral predictors of COVID-19 vaccine hesitancy in Pakistan. J. Multidiscip. Healthc. 14 , 2847–2856 (2021).
Alzeer, A. A. et al. The influence of demographics on influenza vaccine awareness and hesitancy among adults visiting educational hospital in Saudi Arabia. Saudi Pharm. J. 29 , 188–193 (2021).
Alzubaidi, H. et al. A mixed-methods study to assess COVID-19 vaccination acceptability among university students in the United Arab Emirates. Hum. Vaccin. Immunother. 17 , 4074–4082 (2021).
Anandraj, J., Krishnamoorthy, Y., Sivanantham, P., Gnanadas, J. & Kar, S. S. Impact of second wave of COVID-19 pandemic on the hesitancy and refusal of COVID-19 vaccination in Puducherry, India: a longitudinal study. Hum. Vaccin. Immunother. 17 , 5024–5029 (2021).
Arvanitis, M. et al. Factors associated with COVID-19 vaccine trust and hesitancy among adults with chronic conditions. Prev. Med. Rep. 24 , 101484 (2021).
Baccolini, V. et al. COVID-19 vaccine hesitancy among Italian university students: a cross-sectional survey during the first months of the vaccination campaign. Vaccines 9 , 1292 (2021).
Bacon, A. M. & Taylor, S. Vaccination hesitancy and conspiracy beliefs in the UK During the SARS-COV-2 (COVID-19) pandemic. Int. J. Behav. Med. 29 , 448–455 (2022).
Badr, H. et al. Overcoming COVID-19 vaccine hesitancy: insights from an online population-based survey in the United States. Vaccines 9 , 1100 (2021).
Batty, G. D., Deary, I. J., Fawns-Ritchie, C., Gale, C. R. & Altschul, D. Pre-pandemic cognitive function and COVID-19 vaccine hesitancy: cohort study. Brain Behav. Immun. 96 , 100–105 (2021).
Benham, J. L. et al. COVID-19 vaccine-related attitudes and beliefs in Canada: national cross-sectional survey and cluster analysis. JMIR Public Health Surveill. 7 , e30424 (2021).
Bolatov, A. K., Seisembekov, T. Z., Askarova, A. Z. & Pavalkis, D. Barriers to COVID-19 vaccination among medical students in Kazakhstan: development, validation, and use of a new COVID-19 vaccine hesitancy scale. Hum. Vaccin. Immunother. 17 , 4982–4992 (2021).
Butter, S., McGlinchey, E., Berry, E. & Armour, C. Psychological, social, and situational factors associated with COVID-19 vaccination intentions: A study of UK key workers and non-key workers. Br. J. Health Psychol. 27 , 13–29 (2022).
Chandani, S. et al. COVID-19 vaccination hesitancy in India: state of the nation and priorities for research. Brain Behav. Immun. Health 18 , 100375 (2021).
Chaudhary, F. A. et al. Factors influencing COVID-19 vaccine hesitancy and acceptance among the Pakistani population. Hum. Vaccin. Immunother. 17 , 3365–3370 (2021).
Chen, H. et al. Health belief model perspective on the control of COVID-19 vaccine hesitancy and the promotion of vaccination in China: web-based cross-sectional study. J. Med. Internet Res. 23 , e29329 (2021).
Cherian, V., Saini, N. K., Sharma, A. K. & Philip, J. Prevalence and predictors of vaccine hesitancy in an urbanized agglomeration of New Delhi, India. J. Public Health 44 , 70–76 (2022).
Contoli, B. et al. What is the willingness to receive vaccination against COVID-19 among the elderly in Italy? Data from the PASSI d’Argento surveillance system. Front. Public Health 9 , 736976 (2021).
Cordina, M., Lauri, M. A. & Lauri, J. Attitudes towards COVID-19 vaccination, vaccine hesitancy and intention to take the vaccine. Pharm. Pract. 19 , 2317 (2021).
Costantino, A. et al. COVID-19 vaccine acceptance among liver transplant recipients. Vaccines 9 , 1314 (2021).
Costantino, A. et al. COVID-19 vaccine: a survey of hesitancy in patients with celiac disease. Vaccines 9 , 511 (2021).
Coughenour, C., Gakh, M., Sharma, M., Labus, B. & Chien, L. C. Assessing determinants of COVID-19 vaccine hesitancy in Nevada. Health Secur. 19 , 592–604 (2021).
Crane, M. A., Faden, R. R. & Romley, J. A. Disparities in county COVID-19 vaccination rates linked to disadvantage and hesitancy. Health Aff. 40 , 1792–1796 (2021).
Danabal, K. G. M., Magesh, S. S., Saravanan, S. & Gopichandran, V. Attitude towards COVID 19 vaccines and vaccine hesitancy in urban and rural communities in Tamil Nadu, India - a community based survey. BMC Health Serv. Res. 21 , 994 (2021).
Doherty, I. A. et al. COVID-19 vaccine hesitancy in underserved communities of North Carolina. PLoS ONE 16 , e0248542 (2021).
Du, F. et al. Access to vaccination information and confidence/hesitancy towards childhood vaccination: a cross-sectional survey in China. Vaccines 9 , 201 (2021).
Du, M., Tao, L. & Liu, J. The association between risk perception and COVID-19 vaccine hesitancy for children among reproductive women in China: an online survey. Front. Med. 8 , 741298 (2021).
Ebrahimi, O. V. et al. Risk, trust, and flawed assumptions: vaccine hesitancy during the COVID-19 pandemic. Front. Public Health 9 , 700213 (2021).
Ehde, D. M., Roberts, M. K., Humbert, A. T., Herring, T. E. & Alschuler, K. N. COVID-19 vaccine hesitancy in adults with multiple sclerosis in the United States: a follow up survey during the initial vaccine rollout in 2021. Mult. Scler. Relat. Disord. 54 , 103163 (2021).
Ekstrand, M. L. et al. COVID-19 vaccine hesitancy among PLWH in South India: implications for vaccination campaigns. J. Acquir. Immune Defic. Syndr. 88 , 421–425 (2021).
Elizondo-Alzola, U. et al. Vaccine hesitancy among paediatric nurses: prevalence and associated factors. PLoS ONE 16 , e0251735 (2021).
Emerson, E. et al. Vaccine hesitancy among working-age adults with/without disability in the UK. Public Health 200 , 106–108 (2021).
Epstein, S. et al. Vaccination Against SARS-CoV-2 in neuroinflammatory disease: early safety/tolerability data. Mult. Scler. Relat. Disord. 57 , 103433 (2021).
Eyllon, M. et al. Associations between psychiatric morbidity and COVID-19 vaccine hesitancy: an analysis of electronic health records and patient survey. Psychiatry Res. 307 , 114329 (2022).
Fares, S., Elmnyer, M. M., Mohamed, S. S. & Elsayed, R. COVID-19 vaccination perception and attitude among healthcare workers in Egypt. J. Prim. Care Community Health 12 , 21501327211013303 (2021).
Fayed, A. A. et al. Willingness to receive the COVID-19 and seasonal influenza vaccines among the Saudi population and vaccine uptake during the initial stage of the national vaccination campaign: a cross-sectional survey. Vaccines 9 , 765 (2021).
Fazel, M. et al. Willingness of children and adolescents to have a COVID-19 vaccination: results of a large whole schools survey in England. eClinicalMedicine 40 , 101144 (2021).
Fernández-Penny, F. E. et al. COVID-19 vaccine hesitancy among patients in two urban emergency departments. Acad. Emerg. Med . 28 , 1100–1107 (2021).
Fisher, K. A. et al. Preferences for COVID-19 vaccination information and location: associations with vaccine hesitancy, race and ethnicity. Vaccine 39 , 6591–6594 (2021).
Gatwood, J., McKnight, M., Fiscus, M., Hohmeier, K. C. & Chisholm-Burns, M. Factors influencing likelihood of COVID-19 vaccination: a survey of Tennessee adults. Am. J. Health Syst. Pharm. 78 , 879–889 (2021).
Gbeasor-Komlanvi, F. A. et al. Prevalence and factors associated with COVID-19 vaccine hesitancy in health professionals in Togo, 2021. Public Health Pract. 2 , 100220 (2021).
Gendler, Y. & Ofri, L. Investigating the influence of vaccine literacy, vaccine perception and vaccine hesitancy on Israeli parents’ acceptance of the COVID-19 vaccine for their children: a cross-sectional study. Vaccines 9 (2021).
Gentile, A. et al. Vaccine hesitancy in Argentina: validation of WHO scale for parents. Vaccine 39 , 4611–4619 (2021).
Gerretsen, P. et al. Individual determinants of COVID-19 vaccine hesitancy. PLoS ONE 16 , e0258462 (2021).
Gerretsen, P. et al. Vaccine hesitancy is a barrier to achieving equitable herd immunity among racial minorities. Front. Med. 8 , 668299 (2021).
Griva, K. et al. Evaluating rRates and determinants of COVID-19 vaccine hesitancy for adults and children in the Singapore population: Strengthening Our Community’s Resilience against Threats from Emerging Infections (SOCRATEs) Cohort. Vaccines 9 , 1415 (2021).
Guaraldi, F. et al. Rate and predictors of hesitancy toward SARS-CoV-2 vaccine among type 2 diabetic patients: results from an Italian survey. Vaccines 9 , 460 (2021).
Hara, M., Ishibashi, M., Nakane, A., Nakano, T. & Hirota, Y. Differences in COVID-19 vaccine acceptance, hesitancy, and confidence between healthcare workers and the general population in Japan. Vaccines 9 , 1389 (2021).
Holeva, V., Parlapani, E., Nikopoulou, V. A., Nouskas, I. & Diakogiannis, I. COVID-19 vaccine hesitancy in a sample of Greek adults. Psychol. Health Med. 27 , 113–119 (2022).
Hong, J. et al. Knowledge about, attitude and acceptance towards, and predictors of intention to receive the COVID-19 vaccine among cancer patients in eastern China: a cross-sectional survey. J. Integr. Med. 20 , 34–44 (2021).
Hossain, M. B. et al. COVID-19 vaccine hesitancy among the adult population in Bangladesh: a nationwide cross-sectional survey. PLoS ONE 16 , e0260821 (2021).
Hossain, M. B. et al. Health belief model, theory of planned behavior, or psychological antecedents: what predicts covid-19 vaccine hesitancy better among the Bangladeshi adults? Front. Public Health 9 , 711066 (2021).
Huang, H. et al. COVID-19 vaccine uptake, acceptance, and hesitancy among persons with mental disorders during the second stage of China’s nationwide vaccine rollout. Front. Med. 8 , 761601 (2021).
Hussain, S. et al. Perceptions regarding COVID-19 vaccination among a representative Pakistani population coming to tertiary care cardiac hospital. Cureus 13 , e18654 (2021).
Hwang, S. E., Kim, W. H. & Heo, J. Socio-demographic, psychological, and experiential predictors of COVID-19 vaccine hesitancy in South Korea, October–December 2020. Hum. Vaccin. Immunother. 18 , 1–8 (2021).
Hyland, P. et al. Detecting and describing stability and change in COVID-19 vaccine receptibility in the United Kingdom and Ireland. PLoS ONE 16 , e0258871 (2021).
Issanov, A., Akhmetzhanova, Z., Riethmacher, D. & Aljofan, M. Knowledge, attitude, and practice toward COVID-19 vaccination in Kazakhstan: a cross-sectional study. Hum. Vaccin. Immunother. 17 , 3394–3400 (2021).
Jain, J. et al. COVID-19 vaccine hesitancy among medical students in India. Epidemiol. Infect. 149 , e132 (2021).
Kanyike, A. M. et al. Acceptance of the coronavirus disease-2019 vaccine among medical students in Uganda. Trop. Med. Health 49 , 37 (2021).
Kateeb, E. et al. Predictors of willingness to receive COVID-19 vaccine: cross-sectional study of Palestinian dental students. Vaccines 9 , 954 (2021).
Khairat, S., Zou, B. & Adler-Milstein, J. Factors and reasons associated with low COVID-19 vaccine uptake among highly hesitant communities in the US. Am. J. Infect. Control 50 , 262–267 (2022).
Khan, M. S. R., Watanapongvanich, S. & Kadoya, Y. COVID-19 vaccine hesitancy among the younger generation in Japan. Int. J. Environ. Res. Public Health 18 , 11702 (2021).
Khodadadi, A. B., Hansen, B., Kim, Y. I. & Scarinci, I. C. Latinx immigrant mothers’ perceived self-efficacy and intentions regarding human papillomavirus vaccination of their daughters. Womens Health Issues 32 , 293–300 (2022).
King, W. C., Rubinstein, M., Reinhart, A. & Mejia, R. Time trends, factors associated with, and reasons for COVID-19 vaccine hesitancy: a massive online survey of US adults from January–May 2021. PLoS ONE 16 , e0260731 (2021).
King, W. C., Rubinstein, M., Reinhart, A. & Mejia, R. COVID-19 vaccine hesitancy January–May 2021 among 18–64 year old US adults by employment and occupation. Prev. Med. Rep. 24 , 101569 (2021).
Ko, T., Dendle, C., Woolley, I., Morand, E. & Antony, A. SARS-COV-2 vaccine acceptance in patients with rheumatic diseases: a cross-sectional study. Hum. Vaccin. Immunother. 17 , 4048–4056 (2021).
Kollamparambil, U., Oyenubi, A. & Nwosu, C. COVID19 vaccine intentions in South Africa: health communication strategy to address vaccine hesitancy. BMC Public Health 21 , 2113 (2021).
Kozak, A. & Nienhaus, A. COVID-19 vaccination: status and willingness to be vaccinated among employees in health and welfare care in Germany. Int. J. Environ. Res. Public Health 18 , 6688 (2021).
Krishnamurthy, K. et al. COVID-19 vaccine intent among health care professionals of Queen Elizabeth Hospital, Barbados. J. Multidiscip. Healthc. 14 , 3309–3319 (2021).
Ku, L. The association of social factors and health insurance coverage with COVID-19 vaccinations and hesitancy, July 2021. J. Gen. Intern. Med. 37 , 409–414 (2022).
Kuhn, R. et al. COVID-19 vaccine access and attitudes among people experiencing homelessness from pilot mobile phone survey in Los Angeles, CA. PLoS ONE 16 , e0255246 (2021).
Kumar, R., Alabdulla, M., Elhassan, N. M. & Reagu, S. M. Qatar healthcare workers’ COVID-19 vaccine hesitancy and attitudes: a national cross-sectional survey. Front. Public Health 9 , 727748 (2021).
Lang, R. et al. COVID-19 vaccine attitudes and beliefs: a Canadian national cross-sectional survey and cluster analysis. JMIR Public Health Surveill. 7 , e30424 (2021).
Lee, M. & You, M. Direct and indirect associations of media use with COVID-19 vaccine hesitancy in South Korea: cross-sectional web-based survey. J. Med. Internet Res. 24 , e32329 (2022).
Li, M. et al. Hesitancy toward COVID-19 vaccines among medical students in Southwest China: a cross-sectional study. Hum. Vaccin. Immunother. 17 , 4021–4027 (2021).
Liddell, B. J. et al. Factors associated with COVID-19 vaccine hesitancy amongst refugees in Australia. Eur. J. Psychotraumatol. 12 , 1997173 (2021).
Lim, L. J., Lim, A. J. W., Fong, K. K. & Lee, C. G. Sentiments regarding COVID-19 vaccination among graduate students in Singapore. Vaccines 9 , 1141 (2021).
Liu, R. & Li, G. M. Hesitancy in the time of coronavirus: temporal, spatial, and sociodemographic variations in COVID-19 vaccine hesitancy. SSM Popul. Health 15 , 100896 (2021).
Longchamps, C. et al. COVID-19 vaccine hesitancy among persons living in homeless shelters in France. Vaccine 39 , 3315–3318 (2021).
Luk, T. T. et al. Prevalence and determinants of SARS-CoV-2 vaccine hesitancy in Hong Kong: a population-based survey. Vaccine 39 , 3602–3607 (2021).
Luo, H. et al. Willingness to get a COVID-19 vaccine and reasons for hesitancy among medicare beneficiaries: results from a national survey. J. Public Health Manage. Pract. 28 , 70–76 (2022).
Magon, A. et al. The effect of health literacy on vaccine hesitancy among Italian anticoagulated population during COVID-19 pandemic: the moderating role of health engagement. Hum. Vaccin. Immunother. 17 , 5007–5012 (2021).
Martinelli, M. & Veltri, G. A. Do cognitive styles affect vaccine hesitancy? A dual-process cognitive framework for vaccine hesitancy and the role of risk perceptions. Soc. Sci. Med . 289 , 114403 (2021).
McCabe, S. D. et al. Unraveling Attributes of COVID-19 vaccine hesitancy and uptake in the U.S.: a large nationwide study. Preprint at medRxiv https://doi.org/10.1101/2021.04.05.21254918 (2021).
McCarthy, M., Murphy, K., Sargeant, E. & Williamson, H. Examining the relationship between conspiracy theories and covid-19 vaccine hesitancy: a mediating role for perceived health threats, trust, and anomie? Anal. Soc. Issues Public Policy 22 , 106–129 (2022).
McElfish, P. A. et al. Sociodemographic determinants of COVID-19 vaccine hesitancy, fear of infection, and protection self-efficacy. J. Prim. Care Community Health 12 , 21501327211040746 (2021).
Milošević Đorđević, J., Mari, S., Vdović, M. & Milošević, A. Links between conspiracy beliefs, vaccine knowledge, and trust: anti-vaccine behavior of Serbian adults. Soc. Sci. Med . 277 , 113930 (2021).
Mohamad, O. et al. Factors associated with the intention of Syrian adult population to accept COVID19 vaccination: a cross-sectional study. BMC Public Health 21 , 1310 (2021).
Mohan, S., Reagu, S., Lindow, S. & Alabdulla, M. COVID-19 vaccine hesitancy in perinatal women: a cross sectional survey. J. Perinat. Med. 49 , 678–685 (2021).
Mohanan, L., Klugar, M., Pokorna, A. & Riad, A. COVID-19 vaccine booster hesitancy (VBH) of healthcare workers in Czechia: national cross-sectional study. Vaccines 9 , 1437 (2021).
Momplaisir, F. M. et al. Racial/ethnic differences in COVID-19 vaccine hesitancy among health care workers in 2 large academic hospitals. JAMA Netw. Open 4 , e2121931 (2021).
Monami, M. et al. COVID-19 vaccine hesitancy and early adverse events reported in a cohort of 7,881 Italian physicians. Ann. Ig. 34 , 344–357 (2022).
Moore, D. et al. Low COVID-19 vaccine hesitancy in Brazil. Vaccine 39 , 6262–6268 (2021).
Morris, J. L., Baniak, L. M., Luyster, F. S. & Dunbar-Jacob, J. Covid-19 vaccine confidence and hesitancy in nursing students and faculty at a large academic medical center. Nurs. Outlook 70 , 347–354 (2022).
Muhajarine, N. et al. COVID-19 vaccine hesitancy and refusal and associated factors in an adult population in Saskatchewan, Canada: evidence from predictive modelling. PLoS ONE 16 , e0259513 (2021).
Myers, A., Ipsen, C. & Lissau, A. COVID-19 vaccination hesitancy among Americans with disabilities aged 18-65: An exploratory analysis. Disabil. Health J. 15 , 101223 (2022).
Nowak, S. A., Gidengil, C. A., Parker, A. M. & Matthews, L. J. Association among trust in health care providers, friends, and family, and vaccine hesitancy. Vaccine 39 , 5737–5740 (2021).
Okubo, R., Yoshioka, T., Ohfuji, S., Matsuo, T. & Tabuchi, T. COVID-19 vaccine hesitancy and its associated factors in Japan. Vaccines 9 , 662 (2021).
Oliveira, B. et al. Prevalence and factors associated with covid-19 vaccine hesitancy in Maranhão, Brazil. Rev. Saude Publica 55 , 12 (2021).
Omar, D. I. & Hani, B. M. Attitudes and intentions towards COVID-19 vaccines and associated factors among Egyptian adults. J. Infect. Public Health 14 , 1481–1488 (2021).
Orangi, S. et al. Assessing the level and determinants of COVID-19 vaccine confidence in Kenya. Vaccines 9 , 936 (2021).
Pal, S. et al. COVID-19 vaccine hesitancy and attitude toward booster doses among US healthcare workers. Vaccines 9 , 1358 (2021).
Park, H. K., Ham, J. H., Jang, D. H., Lee, J. Y. & Jang, W. M. Political ideologies, government trust, and COVID-19 vaccine hesitancy in South Korea: a cross-sectional survey. Int. J. Environ. Res. Public Health 18 , 10655 (2021).
Piltch-Loeb, R. et al. COVID-19 vaccine concerns about safety, effectiveness, and policies in the United States, Canada, Sweden, and Italy among unvaccinated individuals. Vaccines 9 , 1138 (2021).
Purnell, M. et al. Exploring COVID-19 vaccine hesitancy at a rural historically black college and university. J. Am. Pharm. Assoc. 62 , 340–344 (2021).
Purvis, R. S. et al. Trusted sources of COVID-19 vaccine information among hesitant adopters in the United States. Vaccines 9 , 1418 (2021).
Qunaibi, E. A., Helmy, M., Basheti, I. & Sultan, I. A high rate of COVID-19 vaccine hesitancy in a large-scale survey on Arabs. eLife 10 , e68038 (2021).
Riad, A. et al. Prevalence and drivers of COVID-19 vaccine hesitancy among Czech university students: national cross-sectional study. Vaccines 9 , 948 (2021).
Lavoie, K. et al. Understanding national trends in COVID-19 vaccine hesitancy in Canada - April 2020 to March 2021. BMJ Open 12 , e059411 (2022).
Robertson, E. et al. Predictors of COVID-19 vaccine hesitancy in the UK household longitudinal study. Brain Behav. Immun. 94 , 41–50 (2021).
Rodriguez, R. M. et al. The rapid evaluation of COVID-19 vaccination in emergency departments for underserved patients study. Ann. Emerg. Med. 78 , 502–510 (2021).
Saluja, S. et al. Disparities in COVID-19 vaccine hesitancy among Los Angeles County adults after vaccine authorization. Prev. Med. Rep. 24 , 101544 (2021).
Shallal, A. et al. Evaluation of COVID-19 vaccine attitudes among Arab American healthcare professionals living in the United States. Vaccines 9 , 942 (2021).
Sirikalyanpaiboon, M. et al. COVID-19 vaccine acceptance, hesitancy, and determinants among physicians in a university-based teaching hospital in Thailand. BMC Infect. Dis. 21 , 1174 (2021).
Solak, Ç., Peker-Dural, H., Karlıdağ, S. & Peker, M. Linking the behavioral immune system to COVID-19 vaccination intention: the mediating role of the need for cognitive closure and vaccine hesitancy. Pers. Individ. Dif. 185 , 111245 (2022).
Spinewine, A. et al. Attitudes towards COVID-19 vaccination among hospital staff-understanding what matters to hesitant people. Vaccines 9 , 469 (2021).
Stojanovic, J. et al. Global trends and correlates of COVID-19 vaccination hesitancy: findings from the iCARE study. Vaccines 9 , 661 (2021).
Strathdee, S. A. et al. Correlates of coronavirus disease 2019 (COVID-19) vaccine hesitancy among people who inject drugs in the San Diego-Tijuana border region. Clin. Infect. Dis. https://doi.org/10.1093/cid/ciab975 (2021).
Szilagyi, P. G. et al. The role of trust in the likelihood of receiving a COVID-19 vaccine: results from a national survey. Prev. Med. 153 , 106727 (2021).
Szilagyi, P. G. et al. Likelihood of COVID-19 vaccination by subgroups across the US: post-election trends and disparities. Hum. Vaccin. Immunother. 17 , 3262–3267 (2021).
Tahir, A. I. et al. COVID-19 vaccine acceptance, hesitancy and refusal among Iraqi Kurdish population. Int. J. Health Sci. 16 , 10–16 (2022).
Teasdale, C. A. et al. Parental plans to vaccinate children for COVID-19 in New York city. Vaccine 39 , 5082–5086 (2021).
Thanapluetiwong, S., Chansirikarnjana, S., Sriwannopas, O., Assavapokee, T. & Ittasakul, P. Factors associated with COVID-19 vaccine hesitancy in Thai seniors. Patient Prefer. Adherence 15 , 2389–2403 (2021).
Tsai, R. et al. COVID-19 vaccine hesitancy and acceptance among individuals with cancer, autoimmune diseases, or other serious comorbid conditions: cross-sectional, internet-based survey. JMIR Public Health Surveill. 8 , e29872 (2022).
Uzochukwu, I. C. et al. COVID-19 vaccine hesitancy among staff and students in a Nigerian tertiary educational institution. Ther. Adv. Infect. Dis. 8 , 20499361211054923 (2021).
Vieira Rezende, R. P. et al. Characteristics associated with COVID-19 vaccine hesitancy: a nationwide survey of 1000 patients with immune-mediated inflammatory diseases. Vaccine 39 , 6454–6459 (2021).
Wang, S. X., Bell-Rogers, N., Dillard, D. & Harrington, M. A. COVID-19 vaccine hesitancy in Delaware’s underserved communities. Dela. J. Public Health 7 , 168–175 (2021).
Wang, X., Lin, L., Xu, J., Wang, W. & Zhou, X. Expectant parents’ vaccine decisions influenced by the 2018 Chinese vaccine crisis: a cross-sectional study. Prev. Med. 145 , 106423 (2021).
Waring, M. E. et al. Factors associated with mothers’ hesitancy to receive a COVID-19 vaccine. J. Behav. Med . https://doi.org/10.1007/s10865-021-00268-0 (2022).
Waters, A. R. et al. COVID-19 vaccine hesitancy among adolescent and young adult cancer survivors. JNCI Cancer Spectr. 5 , pkab049 (2021).
West, H. et al. COVID-19 vaccine hesitancy among temporary foreign workers from Bangladesh. Health Syst. Reform 7 , e1991550 (2021).
Willis, D. E. et al. COVID-19 vaccine hesitancy: race/ethnicity, trust, and fear. Clin. Transl. Sci. 14 , 2200–2207 (2021).
Willis, D. E., Presley, J., Williams, M., Zaller, N. & McElfish, P. A. COVID-19 vaccine hesitancy among youth. Hum. Vaccin. Immunother. 17 , 5013–5015 (2021).
Wiysonge, C. S. et al. COVID-19 vaccine acceptance and hesitancy among healthcare workers in South Africa. Expert Rev. Vaccines 21 , 549–559 (2022).
Wu, H., Ward, M., Brown, A., Blackwell, E. & Umer, A. COVID-19 vaccine intent in Appalachian patients with multiple sclerosis. Mult. Scler. Relat. Disord. 57 , 103450 (2021).
Wu, J. et al. COVID-19 vaccine hesitancy among Chinese population: a large-scale national study. Front. Immunol. 12 , 781161 (2021).
Xu, Y. et al. A cross-sectional survey on covid-19 vaccine hesitancy among parents from Shandong vs. Zhejiang. Front. Public Health 9 , 779720 (2021).
Zhang, M. X., Lin, X. Q., Chen, Y., Tung, T. H. & Zhu, J. S. Determinants of parental hesitancy to vaccinate their children against COVID-19 in China. Expert Rev. Vaccines 20 , 1339–1349 (2021).
Zhang, P. et al. Who is more likely to hesitate to accept COVID-19 vaccine: a cross-sectional survey in China. Expert Rev. Vaccines 21 , 397–406 (2022).
Zhao, Y. M. et al. Public willingness and determinants of COVID-19 vaccination at the initial stage of mass vaccination in China. Vaccines 9 , 1172 (2021).
Opel, D. J. et al. Validity and reliability of a survey to identify vaccine-hesitant parents. Vaccine 29 , 6598–6605 (2011).
Abd Halim, H., Abdul-Razak, S., Md Yasin, M. & Isa, M. R. Validation study of the Parent Attitudes About Childhood Vaccines (PACV) questionnaire: the Malay version. Hum. Vaccin. Immunother. 16 , 1040–1049 (2020).
Al-Regaiey, K. A. et al. Influence of social media on parents’ attitudes towards vaccine administration. Hum. Vaccin. Immunother. 18 , 1872340 (2021).
Alsuwaidi, A. R. et al. Vaccine hesitancy and its determinants among Arab parents: a cross-sectional survey in the United Arab Emirates. Hum. Vaccin. Immunother. 16 , 3163–3169 (2020).
Bagateli, L. E. et al. COVID-19 vaccine hesitancy among parents of children and adolescents living in Brazil. Vaccines 9 , 1115 (2021).
Beck, A., Bianchi, A. & Showalter, D. Evidence-based practice model to increase human papillomavirus vaccine uptake: a stepwise approach. Nurs. Womens Health 25 , 430–436 (2021).
Bianco, A., Mascaro, V., Zucco, R. & Pavia, M. Parent perspectives on childhood vaccination: how to deal with vaccine hesitancy and refusal? Vaccine 37 , 984–990 (2019).
Bonsu, N. E. M. et al. Understanding vaccine hesitancy among parents of children with autism spectrum disorder and parents of children with non-autism developmental delays. J. Child Neurol. 36 , 911–918 (2021).
Bulun, M. A. & Acuner, D. Turkish adaptation and reliability and validity study of parent attitudes about childhood vaccines survey. J. Pediatr. Res. 7 , 323–330 (2020).
Chang, J. & Kochel, R. Vaccine hesitancy and attributions for autism among racially and ethnically diverse groups of parents of children with autism spectrum disorder: a pilot study. Autism Res. 13 , 1790–1796 (2020).
Chung-Delgado, K., Valdivia Venero, J. E. & Vu, T. M. Vaccine hesitancy: characteristics of the refusal of childhood vaccination in a Peruvian population. Cureus 13 , e14105 (2021).
Clayton, K., Finley, C., Flynn, D. J., Graves, M. & Nyhan, B. Evaluating the effects of vaccine messaging on immunization intentions and behavior: evidence from two randomized controlled trials in Vermont. Vaccine 39 , 5909–5917 (2021).
Cole, J., Berman, S., Gardner, J., McGuire, K. & Chen, A. M. H. Implementation of a motivational interviewing-based decision tool to improve childhood vaccination rates: pilot study protocol. Res. Social Adm. Pharm. 17 , 619–624 (2020).
Cunningham, R. M., Guffey, D., Minard, C. G., Opel, D. J. & Boom, J. A. The effect of screening for vaccine hesitancy on the subsequent development of hesitancy: a randomized controlled trial, Houston, TX. Hum. Vaccin. Immunother. 17 , 1994–2000 (2021).
Daley, M. F., Narwaney, K. J., Shoup, J. A., Wagner, N. M. & Glanz, J. M. Addressing parents’ vaccine concerns: a randomized trial of a social media intervention. Am. J. Prev. Med . 55 , 44–54 (2018).
Eby, A. Z. Impacting parental vaccine decision-making. Pediatr. Nurs. 43 , 22–29, 34 (2017).
Goin-Kochel, R. P. et al. Beliefs about causes of autism and vaccine hesitancy among parents of children with autism spectrum disorder. Vaccine 38 , 6327–6333 (2020).
Henrikson, N. B. et al. Longitudinal trends in vaccine hesitancy in a cohort of mothers surveyed in Washington State, 2013–2015. Public Health Rep. 132 , 451–454 (2017).
Henrikson, N. B. et al. Physician communication training and parental vaccine hesitancy: a randomized trial. Pediatrics 136 , 70–79 (2015).
Hofstetter, A. M. et al. Parental vaccine hesitancy and declination of influenza vaccination among hospitalized children. Hosp. Pediatr. 8 , 628–635 (2018).
Kalok, A. et al. Vaccine hesitancy towards childhood immunisation amongst urban pregnant mothers in Malaysia. Vaccine 38 , 2183–2189 (2020).
Langkamp, D. L., Dusseau, A. & Brown, M. F. Vaccine hesitancy and low immunization rates in children with Down Syndrome. J. Pediatr. 223 , 64–67 (2020).
Mical, R., Martin-Velez, J., Blackstone, T. & Derouin, A. Vaccine hesitancy in rural pediatric primary care. J. Pediatr. Health Care 35 , 16–22 (2020).
Mohd Azizi, F. S., Kew, Y. & Moy, F. M. Vaccine hesitancy among parents in a multi-ethnic country, Malaysia. Vaccine 35 , 2955–2961 (2017).
Napolitano, F., D’Alessandro, A. & Angelillo, I. F. Investigating Italian parents’ vaccine hesitancy: a cross-sectional survey. Hum. Vaccin. Immunother. 14 , 1558–1565 (2018).
Olarewaju, V. O. et al. Application of the Parent Attitudes about Childhood Vaccines (PACV) survey in three national languages in Switzerland: exploratory factor analysis and Mokken scale analysis. Hum. Vaccin. Immunother. 17 , 2652–2660 (2021).
Opel, D. J. et al. The architecture of provider-parent vaccine discussions at health supervision visits. Pediatrics 132 , 1037–1046 (2013).
Opel, D. J. et al. The relationship between parent attitudes about childhood vaccines survey scores and future child immunization status: a validation study. JAMA Pediatr. 167 , 1065–1071 (2013).
Opel, D. J. et al. Impact of childhood vaccine discussion format over time on immunization status. Acad. Pediatr. 18 , 430–436 (2018).
Orr, C. & Beck, A. F. Measuring vaccine hesitancy in a minority community. Clin. Pediatr. 56 , 784–788 (2017).
Reuben, R., Aitken, D., Freedman, J. L. & Einstein, G. Mistrust of the medical profession and higher disgust sensitivity predict parental vaccine hesitancy. PLoS ONE 15 , e0237755 (2020).
Sabahelzain, M. M., van den Borne, B., Moukhyer, M. & Bosma, H. Determinants of measles vaccine hesitancy among Sudanese parents in Khartoum State, Sudan: a cross-sectional study. Vaccines 10 , 6 (2022).
Sahni, L. C. et al. Vaccine hesitancy and illness perceptions: comparing parents of children with autism spectrum disorder to other parent groups. Child. Health Care 35 , 385–402 (2020).
Sankaranarayanan, S., Jayaraman, A. & Gopichandran, V. Assessment of vaccine hesitancy among parents of children between 1 and 5 years of age at a tertiary care hospital in Chennai. Indian J. Community Med. 44 , 394–396 (2019).
Strelitz, B. et al. Parental vaccine hesitancy and acceptance of seasonal influenza vaccine in the pediatric emergency department. Vaccine 33 , 1802–1807 (2015).
Wagner, A. L. et al. Vaccine hesitancy and concerns about vaccine safety and effectiveness in Shanghai, China. Am. J. Prev. Med . 60 , S77–S86 (2020).
Williams, J. T. B., Rice, J. D. & O’Leary, S. T. Associations between religion, religiosity, and parental vaccine hesitancy. Vaccine X 9 , 100121 (2021).
Williams, S. E. et al. Screening tool predicts future underimmunization among a pediatric practice in Tennessee. Clin. Pediatr. 55 , 537–542 (2016).
Williams, S. E. et al. A randomized trial to increase acceptance of childhood vaccines by vaccine-hesitant parents: a pilot study. Acad. Pediatr. 13 , 475–480 (2013).
Yufika, A. et al. Parents’ hesitancy towards vaccination in Indonesia: a cross-sectional study in Indonesia. Vaccine 38 , 2592–2599 (2020).
Akhmetzhanova, Z., Sazonov, V., Riethmacher, D. & Aljofan, M. Vaccine adherence: the rate of hesitancy toward childhood immunization in Kazakhstan. Expert Rev. Vaccines 19 , 579–584 (2020).
Cunningham, R. M. et al. Development of a Spanish version of the parent attitudes about childhood vaccines survey. Hum. Vaccin. Immunother. 15 , 1106–1110 (2019).
Cunningham, R. M. et al. Prevalence of vaccine hesitancy among expectant mothers in Houston, Texas. Acad. Pediatr. 18 , 154–160 (2018).
Della Polla, G., Pelullo, C. P., Napolitano, F. & Angelillo, I. F. HPV vaccine hesitancy among parents in Italy: a cross-sectional study. Hum. Vaccin. Immunother. 16 , 2744–2751 (2020).
Dubé, E. et al. Overview of knowledge, attitudes, beliefs, vaccine hesitancy and vaccine acceptance among mothers of infants in Quebec, Canada. Hum. Vaccin. Immunother. 15 , 113–120 (2019).
Marshall, S., Moore, A. C., Sahm, L. J. & Fleming, A. Parent attitudes about childhood vaccines: point prevalence survey of vaccine hesitancy in an Irish population. Pharmacy 9 , 188 (2021).
Moyer-Gusé, E., Robinson, M. J. & McKnight, J. The role of humor in messaging about the MMR vaccine. J. Health Commun. 23 , 514–522 (2018).
Olarewaju, V. O. et al. The Youth Attitudes about Vaccines (YAV-5) scale: adapting the parent attitudes about childhood vaccines short scale for use with youth in German, French, and Italian in Switzerland, exploratory factor analysis and mokken scaling analysis. Hum. Vaccin. Immunother. 17 , 5183–5190 (2021).
Roberts, J. R. et al. Vaccine hesitancy among parents of adolescents and its association with vaccine uptake. Vaccine 33 , 1748–1755 (2015).
Ruggiero, K. M. et al. Parents’ intentions to vaccinate their children against COVID-19. J. Pediatr. Health Care 35 , 509–517 (2021).
Voo, J. Y. H. et al. Vaccine knowledge, awareness and hesitancy: a cross sectional survey among parents residing at Sandakan District, Sabah. Vaccines 9 , 1348 (2021).
Wallace, A. S. et al. Development of a valid and reliable scale to assess parents’ beliefs and attitudes about childhood vaccines and their association with vaccination uptake and delay in Ghana. Vaccine 37 , 848–856 (2019).
Wang, Y. & Zhang, X. Influence of parental psychological flexibility on pediatric COVID-19 vaccine hesitancy: mediating role of self-efficacy and coping style. Front. Psychol. 12 , 783401 (2021).
Whelan, S. O., Moriarty, F., Lawlor, L., Gorman, K. M. & Beamish, J. Vaccine hesitancy and reported non-vaccination in an Irish pediatric outpatient population. Eur. J. Pediatr. 180 , 2839–2847 (2021).
Williams, J. T. B. & O’Leary, S. T. Denver religious leaders’ vaccine attitudes, practices, and congregational experiences. J. Relig. Health 58 , 1356–1367 (2019).
Yörük, S. & Güler, D. Factors associated with pediatric vaccine hesitancy of parents: a cross-sectional study in Turkey. Hum. Vaccin. Immunother. 17 , 4505–4511 (2021).
Zhang, H. et al. Vaccine hesitancy among parents and its influencing factors: a cross-sectional study in Guangzhou, China. Hum. Vaccin. Immunother. 17 , 5153–5161 (2021).
Cataldi, J. R., Dempsey, A. F., Allison, M. A. & O’Leary, S. T. Impact of publicly available vaccination rates on parental school and child care choice. Vaccine 36 , 4525–4531 (2018).
Cataldi, J. R., Dempsey, A. F. & O’Leary, S. T. Measles, the media, and MMR: impact of the 2014–15 measles outbreak. Vaccine 34 , 6375–6380 (2016).
Cataldi, J. R. et al. Addressing personal parental values in decisions about childhood vaccination: measure development. Vaccine 37 , 5688–5697 (2019).
McDonald, C. et al. a consent support resource with benefits and harms of vaccination does not increase hesitancy in parents–an acceptability study. Vaccines 8 , 500 (2020).
Opel, D. J. et al. Previsit screening for parental vaccine hesitancy: a cluster randomized trial. Pediatrics 144 , e20190802 (2019).
Williams, J. T. B. et al. Adapting and piloting a vaccine hesitancy questionnaire in rural Guatemala. Vaccine 39 , 180–184 (2021).
Akbas Gunes, N. Parents’ perspectives about vaccine hesitancies and vaccine rejection, in the west of Turkey. J. Pediatr. Nurs. 53 , e186–e194 (2020).
Aldakhil, H., Albedah, N., Alturaiki, N., Alajlan, R. & Abusalih, H. Vaccine hesitancy towards childhood immunizations as a predictor of mothers’ intention to vaccinate their children against COVID-19 in Saudi Arabia. J. Infect. Public Health 14 , 1497–1504 (2021).
AlGoraini, Y. M. et al. Confidence toward vaccination as reported by parents of children admitted to a tertiary care hospital in Riyadh, Saudi Arabia: a cross sectional study. Vacunas 21 , 95–104 (2020).
Alsubaie, S. S. et al. Vaccine hesitancy among Saudi parents and its determinants. Result from the WHO SAGE working group on vaccine hesitancy survey tool. Saudi Med. J. 40 , 1242–1250 (2019).
Connors, J. T., Hodges, E. A., DʼAuria, J. & Windham, L. Implementing vaccine hesitancy screening for targeted education. J. Am. Assoc. Nurse Pract. 30 , 450–459 (2018).
Dasgupta, P., Bhattacherjee, S., Mukherjee, A. & Dasgupta, S. Vaccine hesitancy for childhood vaccinations in slum areas of Siliguri, India. Indian J. Public Health 62 , 253–258 (2018).
Johnson, K. D. et al. Combatting a “twin-demic”: a quantitative assessment of COVID-19 and influenza vaccine hesitancy in primary care patients. Health Promot. Perspect. 11 , 179–185 (2021).
Krishnamoorthy, Y. et al. Factors related to vaccine hesitancy during the implementation of Measles-Rubella campaign 2017 in rural Puducherry–a mixed-method study. J. Fam. Med. Prim. Care 8 , 3962–3970 (2019).
Masters, N. B., Tefera, Y. A., Wagner, A. L. & Boulton, M. L. Vaccine hesitancy among caregivers and association with childhood vaccination timeliness in Addis Ababa, Ethiopia. Hum. Vaccin. Immunother. 14 , 2340–2347 (2018).
Miko, D., Costache, C., Colosi, H. A., Neculicioiu, V. & Colosi, I. A. Qualitative assessment of vaccine hesitancy in Romania. Medicina 55 , 282 (2019).
Sharma, S., Akhtar, F., Singh, R. K. & Mehra, S. Understanding the three As (awareness, access, and acceptability) dimensions of vaccine hesitancy in Odisha, India. Clin. Epidemiol. Glob. Health 8 , 399–403 (2020).
Soysal, G., Durukan, E. & Akdur, R. The evaluation of vaccine hesitancy and refusal for childhood vaccines and the covid-19 vaccine in individuals aged between 18 and 25 years. Turkish J. Immunol. 9 , 120–127 (2021).
Khezong, A. et al. Association between adult vaccine hesitancy and parental acceptance of childhood COVID-19 vaccines: a web-based survey in a northwestern region in China. Vaccines 9 , 1088 (2021).
Akel, K. B., Masters, N. B., Shih, S. F., Lu, Y. & Wagner, A. L. Modification of a vaccine hesitancy scale for use in adult vaccinations in the United States and China. Hum. Vaccin. Immunother. 17 , 2639–2646 (2021).
Chen, M. et al. An online survey of the attitude and willingness of Chinese adults to receive COVID-19 vaccination. Hum. Vaccin. Immunother. 17 , 2279–2288 (2021).
Dolu, İ., Turhan, Z. & Yalnız Dilcen, H. COVID-19 vaccine acceptance is associated with vaccine hesitancy, perceived risk and previous vaccination experiences. Disaster Med. Public Health Prep . https://doi.org/10.1017/dmp.2021.370 (2021).
Domek, G. J. et al. Measuring vaccine hesitancy: field testing the WHO SAGE Working Group on Vaccine Hesitancy survey tool in Guatemala. Vaccine 36 , 5273–5281 (2018).
Gatwood, J. et al. Extent of and reasons for vaccine hesitancy in adults at high-risk for pneumococcal disease. Am. J. Health Promot . 35 , 908–916 (2021).
Han, Y. et al. Parental category B vaccine hesitancy and associated factors in China: an online cross-sectional survey. Expert Rev. Vaccines 21 , 145–153 (2021).
He, K., Mack, W. J., Neely, M., Lewis, L. & Anand, V. Parental perspectives on immunizations: impact of the COVID-19 pandemic on childhood vaccine hesitancy. J. Community Health 47 , 39–52 (2022).
Helmkamp, L. J. et al. A validated modification of the vaccine hesitancy scale for childhood, influenza and HPV vaccines. Vaccine 39 , 1831–1839 (2021).
Jones, D. L. et al. Severe acute respiratory syndrome coronavirus 2: vaccine hesitancy among underrepresented racial and ethnic groups with HIV in Miami, Florida. Open Forum Infect. Dis. 8 , ofab154 (2021).
Kasrine Al Halabi, C. et al. Attitudes of Lebanese adults regarding COVID-19 vaccination. BMC Public Health 21 , 998 (2021).
Kempe, A. et al. Parental hesitancy about routine childhood and influenza vaccinations: a national survey. Pediatrics 146 , e20193852 (2020).
Kim, H. W. et al. Perceptions of nurses on human papillomavirus vaccinations in the Republic of Korea. PLoS ONE 14 , e0211475 (2019).
Liu, P. L., Zhao, X. & Wan, B. COVID-19 information exposure and vaccine hesitancy: the influence of trust in government and vaccine confidence. Psychol. Health Med. https://doi.org/10.1080/13548506.2021.2014910 (2021).
Lu, F. & Sun, Y. COVID-19 vaccine hesitancy: the effects of combining direct and indirect online opinion cues on psychological reactance to health campaigns. Comput. Hum. Behav. 127 , 107057 (2022).
Lu, J. et al. Sensitivity to COVID-19 vaccine effectiveness and safety in Shanghai, China. Vaccines 9 , 472 (2021).
Lu, X. et al. Low willingness to vaccinate against herpes zoster in a Chinese metropolis. Hum. Vaccin. Immunother. 17 , 4163–4170 (2021).
Lu, X. et al. Gap between willingness and behavior in the vaccination against influenza, pneumonia, and herpes zoster among Chinese aged 50–69 years. Expert Rev. Vaccines 20 , 1147–1152 (2021).
Luyten, J., Bruyneel, L. & van Hoek, A. J. Assessing vaccine hesitancy in the UK population using a generalized vaccine hesitancy survey instrument. Vaccine 37 , 2494–2501 (2019).
Önal, Ö., Eroğlu, H. N., Evcil, F. Y., Kişioğlu, A. N. & Uskun, E. Validity and reliability of Turkish version of the Vaccine Hesitancy Scale. Turk. Arch. Pediatr. 56 , 230–235 (2021).
Ren, J. et al. The demographics of vaccine hesitancy in Shanghai, China. PLoS ONE 13 , e0209117 (2018).
Sabahelzain, M. M. et al. Psychometric properties of the adapted measles vaccine hesitancy scale in Sudan. PLoS ONE 15 , e0237171 (2020).
Sadaqat, W. et al. Determination of COVID-19 vaccine hesitancy among university students. Cureus 13 , e17283 (2021).
Shapiro, G. K. et al. The vaccine hesitancy scale: psychometric properties and validation. Vaccine 36 , 660–667 (2018).
Shen, X. et al. Assessing the COVID-19 vaccine hesitancy in the Chinese adults using a generalized vaccine hesitancy survey instrument. Hum. Vaccin. Immunother. 17 , 4005–4012 (2021).
Shih, S. F. et al. Vaccine hesitancy and rejection of a vaccine for the novel coronavirus in the United States. Front. Immunol. 12 , 558270 (2021).
Szilagyi, P. G. et al. Prevalence and characteristics of HPV vaccine hesitancy among parents of adolescents across the US. Vaccine 38 , 6027–6037 (2020).
Temsah, M. H. et al. Parental attitudes and hesitancy about COVID-19 vs. routine childhood vaccinations: a national survey. Front. Public Health 9 , 752323 (2021).
Thaker, J. The persistence of vaccine hesitancy: COVID-19 vaccination intention in New Zealand. J. Health Commun. 26 , 104–111 (2021).
Turhan, Z., Dilcen, H. Y. & Dolu, İ. The mediating role of health literacy on the relationship between health care system distrust and vaccine hesitancy during COVID-19 pandemic. Curr. Psychol . https://doi.org/10.1007/s12144-021-02105-8 (2021).
Vallis, M. & Glazer, S. Protecting individuals living with overweight and obesity: attitudes and concerns toward COVID-19 vaccination in Canada. Obesity 29 , 1128–1137 (2021).
Wagner, A. L. et al. Comparisons of vaccine hesitancy across five low- and middle-income countries. Vaccines 7 , 155 (2019).
Wagner, A. L., Shotwell, A. R., Boulton, M. L., Carlson, B. F. & Mathew, J. L. Demographics of vaccine hesitancy in Chandigarh, India. Front. Med. 7 , 585579 (2020).
Wang, Q. et al. Delays in routine childhood vaccinations and their relationship with parental vaccine hesitancy: a cross-sectional study in Wuxi, China. Expert Rev. Vaccines 21 , 135–143 (2021).
Wang, Q. et al. Vaccine hHesitancy: COVID-19 and influenza vaccine willingness among parents in Wuxi, China–a cross-sectional study. Vaccines 9 , 342 (2021).
Xu, Y. et al. COVID-19 vaccination attitudes with neuromyelitis optica spectrum disorders: vaccine hesitancy and coping style. Front. Neurol. 12 , 717111 (2021).
Yeşiltepe, A., Aslan, S. & Bulbuloglu, S. Investigation of perceived fear of COVID-19 and vaccine hesitancy in nursing students. Hum. Vaccin. Immunother. 17 , 5030–5037 (2021).
Duong, T. V. et al. Oxford COVID-19 vaccine hesitancy in school principals: impacts of gender, well-being, and coronavirus-related health literacy. Vaccines 9 , 958 (2021).
Guaracha-Basáñez, G. et al. COVID-19 vaccine hesitancy among Mexican outpatients with rheumatic diseases. Hum. Vaccin. Immunother. 17 , 5038–5047 (2021).
Joshi, A. et al. COVID-19 vaccine hesitancy in healthcare workers amidst the second wave of the pandemic in India: a single centre study. Cureus 13 , e17370 (2021).
Zhang, X., Guo, Y., Zhou, Q., Tan, Z. & Cao, J. The mediating roles of medical mistrust, knowledge, confidence and complacency of vaccines in the pathways from conspiracy beliefs to vaccine hesitancy. Vaccines 9 , 1342 (2021).
Pomares, T. D. et al. Association of cognitive biases with human papillomavirus vaccine hesitancy: a cross-sectional study. Hum. Vaccin. Immunother. 16 , 1018–1023 (2020).
Ashkenazi, S. et al. The relationship between parental source of information and knowledge about measles / measles vaccine and vaccine hesitancy. Vaccine 38 , 7292–7298 (2020).
Biswas, N., Mustapha, T., Khubchandani, J. & Price, J. H. The nature and extent of COVID-19 vaccination hesitancy in healthcare workers. J. Community Health 46 , 1244–1251 (2021).
Boattini, M. et al. Rubella serosurvey and factors related to vaccine hesitancy in childbearing women in Italy. Prev. Med. Rep. 15 , 100945 (2019).
Browne, S. K. et al. Coronavirus disease 2019 (COVID-19) vaccine hesitancy among physicians, physician assistants, nurse practitioners, and nurses in two academic hospitals in Philadelphia. Infect. Control Hosp. Epidemiol. https://doi.org/10.1017/ice.2021.410 (2021).
Dybsand, L. L., Hall, K. J. & Carson, P. J. Immunization attitudes, opinions, and knowledge of healthcare professional students at two Midwestern universities in the United States. BMC Med. Educ. 19 , 242 (2019).
Gao, X., Li, H., He, W. & Zeng, W. COVID-19 vaccine hesitancy among medical students: the next COVID-19 challenge in Wuhan, China. Disaster Med. Public Health Prep . https://doi.org/10.1017/dmp.2021.291 (2021).
Gerber, J. E. et al. Vaccinomics: a cross-sectional survey of public values. Hum. Vaccin. Immunother. 17 , 2999–3015 (2021).
Giambi, C. et al. Parental vaccine hesitancy in Italy – results from a national survey. Vaccine 36 , 779–787 (2018).
Guay, M., Gosselin, V., Petit, G., Baron, G. & Gagneur, A. Determinants of vaccine hesitancy in Quebec: a large population-based survey. Hum. Vaccin. Immunother. 15 , 2527–2533 (2019).
Harapan, H. et al. Vaccine hesitancy among communities in ten countries in Asia, Africa, and South America during the COVID-19 pandemic. Pathog. Glob. Health 116 , 236–243 (2022).
Hornsey, M. J., Lobera, J. & Díaz-Catalán, C. Vaccine hesitancy is strongly associated with distrust of conventional medicine, and only weakly associated with trust in alternative medicine. Soc. Sci. Med. 255 , 113019 (2020).
Hu, Y., Chen, Y., Liang, H. & Wang, Y. Reliability and validity of a survey to identify vaccine hesitancy among parents in Changxing county, Zhejiang province. Hum. Vaccin. Immunother. 15 , 1092–1099 (2019).
Johnson, D. K. et al. Combating vaccine hesitancy with vaccine-preventable disease familiarization: an interview and curriculum intervention for college students. Vaccines 7 , 39 (2019).
Kassianos, G. et al. Motors of influenza vaccination uptake and vaccination advocacy in healthcare workers: a comparative study in six European countries. Vaccine 36 , 6546–6552 (2018).
Klassen, A. C. et al. Formative research to address vaccine hesitancy in Tajikistan. Vaccine 39 , 1516–1527 (2021).
Liu, Y. et al. COVID-19 vaccination in people living with HIV (PLWH) in China: a cross sectional study of vaccine hesitancy, safety, and immunogenicity. Vaccines 9 , 1458 (2021).
Paoli, S. et al. Assessing vaccine hesitancy among healthcare workers: a cross-sectional study at an Italian paediatric hospital and the development of a healthcare worker’s vaccination compliance index. Vaccines 7 , 201 (2019).
Rahman, M., Hossain, A., Sufian, A. & Anwar, N. COVID-19 vaccine demand, hesitancy, and nationalism: a case of protection motivation behavior in Bangladesh. J. Infect. Dev. Ctries 15 , 1388–1395 (2021).
Raude, J. et al. Opening the ‘Vaccine Hesitancy’ black box: how trust in institutions affects French GPs’ vaccination practices. Expert Rev. Vaccines 15 , 937–948 (2016).
Schwarzinger, M., Watson, V., Arwidson, P., Alla, F. & Luchini, S. COVID-19 vaccine hesitancy in a representative working-age population in France: a survey experiment based on vaccine characteristics. Lancet Public Health 6 , e210–e221 (2021).
Stoeckel, F., Carter, C., Lyons, B. A. & Reifler, J. Association of vaccine hesitancy and immunization coverage rates in the European Union. Vaccine 39 , 3935–3939 (2021).
Tomljenovic, M., Petrovic, G., Antoljak, N. & Hansen, L. Vaccination attitudes, beliefs and behaviours among primary health care workers in northern Croatia. Vaccine 39 , 738–745 (2021).
Verger, P. et al. Vaccine hesitancy among hospital staff physicians: a cross-sectional survey in France in 2019. Vaccine 39 , 4481–4488 (2021).
Verger, P. et al. Vaccine hesitancy among general practitioners and its determinants during controversies: a national cross-sectional survey in France. EBioMedicine 2 , 891–897 (2015).
Carranza, D., Dub, T. & Sivelä, J. Vaccine Hesitancy and Uptake. From Research and Practices to Implementation (Finnish Institute for Health and Welfare (THL), 2021); https://eu-jav.com/public_deliverable/deliverable-8-1-vaccine-hesitancy-and-uptake-from-research-and-practices-to-implementation/
Merriam-Webster Dictionary Hesitancy https://www.merriam-webster.com/dictionary/hesitancy (2021).
Merriam-Webster Dictionary Vaccine Hesitancy https://www.merriam-webster.com/dictionary/vaccine%20hesitancy (2021).
Siani, A. et al. Investigating the determinants of vaccine hesitancy within undergraduate students’ social sphere. Z. Gesundh. Wiss . https://doi.org/10.1007/s10389-021-01538-6 (2021).
Strong, H., Reiter-Purtill, J., Howarth, T., West-Smith, L. & Zeller, M. H. Early COVID-19 vaccine hesitancy characteristics in mothers following bariatric surgery. Obes. Surg. 32 , 852–860 (2022).
Lavail, K. H. & Kennedy, A. M. The role of attitudes about vaccine safety, efficacy, and value in explaining parents’ reported vaccination behavior. Health Educ. Behav. 40 , 544–551 (2013).
Frew, P. M. et al. Development of a measure to assess vaccine confidence among men who have sex with men. Expert Rev. Vaccines 17 , 1053–1061 (2018).
World Bank Country and Lending Groups (The World Bank, 2021); https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups
Hong, Q. N. et al. Mixed Methods Appraisal Tool (MMAT), version 2018 (Canadian Intellectual Property Office, Industry Canada, 2018).
Hong, Q. N. et al. Reporting the Results of the MMAT (Version 2018) http://mixedmethodsappraisaltoolpublic.pbworks.com/w/file/fetch/140056890/Reporting%20the%20results%20of%20the%20MMAT.pdf (2020).
Download references
Acknowledgements
J.L.A.H. and M.E.J.L.H. received funding from The Netherlands Organisation for Health Research and Development (ZonMw project number 839190002). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. We thank J. van Haren for her valuable contribution in sorting and organizing the data of this systematic review.
Author information
Authors and affiliations.
Radboud University Medical Center, Radboud Institute for Health Sciences, Primary and Community Care, Nijmegen, the Netherlands
Daphne Bussink-Voorend, Jeannine L. A. Hautvast & Olga Visser
Behavioural Science Institute, Radboud University, Nijmegen, the Netherlands
Lisa Vandeberg
Radboud University Medical Center, Radboud Institute for Health Sciences, IQ Healthcare, Nijmegen, the Netherlands
Marlies E. J. L. Hulscher
You can also search for this author in PubMed Google Scholar
Contributions
D.B.-V., J.L.A.H., O.V. and M.E.J.L.H. designed the project and analysed the data. D.B.-V., J.L.A.H., L.V., O.V. and M.E.J.L.H. interpreted the data. The manuscript, figures and tables were drafted by D.B.-V. and edited by J.L.A.H., L.V., O.V. and M.E.J.L.H.
Corresponding author
Correspondence to Daphne Bussink-Voorend .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Peer review
Peer review information.
Nature Human Behaviour thanks Chuanxi Fu, Amalie Dyda and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information.
Supplementary methods, PRISMA 2020 for abstracts checklist, PRISMA 2020 checklist.
Reporting Summary
Peer review file, supplementary table 1.
Overview and characteristics of included studies.
Supplementary Table 2
Overview of extracted excerpts.
Supplementary Table 3
Overview of vaccine hesitancy subpopulations.
Supplementary Table 4
Overview of studies describing a measurement of vaccine hesitancy.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Cite this article.
Bussink-Voorend, D., Hautvast, J.L.A., Vandeberg, L. et al. A systematic literature review to clarify the concept of vaccine hesitancy. Nat Hum Behav 6 , 1634–1648 (2022). https://doi.org/10.1038/s41562-022-01431-6
Download citation
Received : 25 November 2021
Accepted : 13 July 2022
Published : 22 August 2022
Issue Date : December 2022
DOI : https://doi.org/10.1038/s41562-022-01431-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
An effective covid-19 vaccine hesitancy intervention focused on the relative risks of vaccination and infection.
- Cameron O’Neill Byerley
Scientific Reports (2024)
Association of personality traits and socio-environmental factors with COVID-19 pandemic-related conspiratorial thinking in the D-A-CH region
- Jakob Weitzer
- Gerald Steiner
SN Social Sciences (2024)
Modelling the impact of opinion flexibility on the vaccination choices during epidemics
- Rossella Della Marca
- Marco Menale
Ricerche di Matematica (2024)
Do peer-based education interventions effectively improve vaccination acceptance? a systematic review
- Elisa L. S. Gobbo
- Claudia Hanson
- Sibylle Herzig van Wees
BMC Public Health (2023)
The association between vaccine hesitancy and pertussis: a systematic review and meta-analysis
- Yuning Wang
- Naiyang Shi
Italian Journal of Pediatrics (2023)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Institute of Medicine (US) Forum on Emerging Infections; Knobler SL, Mahmoud AAF, Pray LA, editors. Biological Threats and Terrorism: Assessing The Science and Response Capabilities: Workshop Summary. Washington (DC): National Academies Press (US); 2002.

Biological Threats and Terrorism: Assessing The Science and Response Capabilities: Workshop Summary.
- Hardcopy Version at National Academies Press
3 Vaccines: Research, Development, Production, and Procurement Issues
Vaccines not only afford the best protection against infectious disease but can serve as strong deterrence factors as well. From a bioterrorist perspective, vaccine-resistant agents are more difficult to engineer than drug-resistant agents. But the potential market has been too small and uncertain to encourage the vaccine industry to make large investments in research, development, and manufacturing of new products. This is alarming considering the eight to ten years often needed to develop a new vaccine, compared to only two to three years to develop a new bioweapon.
Even among the four major vaccine manufacturers, there is insufficient production capacity. It was suggested during this session that in order to move animal and clinical testing forward, incentives need to be established to reduce the current challenges of vaccine development; vaccine production priorities need to be set and a central office or leader authorized to declare top priorities; and the role of the major vaccine manufacturers needs to be facilitated by clear directions and active collaboration between industry and government.
The use of vaccines as a civilian biodefense measure presents multiple challenges that are quite different from those of vaccine use by the military. Much of the challenge is due to the fact that the threats are uncertain and risk-benefit information difficult to assess. The very nature of terrorism produces a high level of uncertainty about what to expect and how to prepare. Additionally, DoD has developed vaccines to be used in normal healthy adults between the ages of 18 and 65, not pediatric, geriatric, immunocompromised or other subsets of the civilian population. Currently, there is no policy in place for immunizing the civilian population as a bioweapons defense measure, however several government agencies are working at unprecedented speed to put the correct policies into place.
The threat of a global pandemic makes smallpox one of the top vaccine priorities. An aggressive clinical development plan is currently in place; its goal is to build the stockpile with enough vaccine to protect the entire country within the year. The vaccine immune globulin (VIG) supply also needs to be expanded. Long-term goals include developing a safer vaccine that can be used in immunocompromised or other at-risk individuals.
Anthrax vaccine is another top priority. As of May 2001, over two million doses of the current anthrax vaccine have been administered to over 500,000 individuals, mostly military personnel. But there is an urgent need for more anthrax vaccine for the immunization of high risk civilian populations, as well as for use in medical management of exposed individuals in conjunction with antibiotics. Currently, there is only one manufacturer of licensed anthrax vaccine, but production is limited because of regulatory problems. Several commercial firms have offered to aid in scaled-up production, but the inherent variability of the manufacturing process and the risk of failure when scaling up so rapidly to such a high volume could create problems. Other mid to long-term anthrax vaccine needs include the development of a second-generation vaccine (e.g., a recombinant protective antigen vaccine) as well as better delivery technologies (e.g., plasmid DNA).
Of lesser importance than vaccines against smallpox and anthrax are vaccines against bacterial infections for which antibiotics can be used and other viral agents that, for the present, seem to be a lesser threat.
A recent independent review of DoD's vaccine acquisition program recommended an integrated approach between DoD and industry and the establishment of a dedicated national vaccine production facility that allows for maximal flexibility and expandable manufacturing capability for the production of various types of vaccines. Whether the proposed facility will be government-owned and contractor-operated or contractor-owned and contractor operated is open for discussion.
Ebola virus provides a useful paradigm for how a molecular-level understanding of the pathogenesis of a virus can be used to develop a new vaccine for an infectious agent that would otherwise be difficult to tackle. This type of molecular genetics approach can reveal possible targets for antiviral drugs as well. For example, recent studies have shown that one of the domains of the ebola virus forms a coil-to-coil structure that is similar to structures found in other viruses, including HIV and influenza. This similarity suggests that the approach being used to develop products for antiviral use against HIV may also be useful for targeting the coil-to-coil region of ebola virus. In fact, targeting this coil-to-coil structure may prove to be a useful general antiviral strategy against many different viruses.
Other vaccine issues that were raised during this session include:
- Improving the usefulness of DNA vaccines, which work well in rodents but not primates.
- Consideration of combination vaccines, for example can we use what we have learned from ebola to make a combination vaccine for use against all hemorrhagic fevers?
- Application of genomics to vaccine research could have, for example if we could use the new high throughput technology to identify genomic biomarkers for vaccine efficacy, then we could use these biomarkers in the future to move forward more quickly toward licensure.
- The need for a strong infrastructure to receive the intense flow of resources that would be expected with a rapid deployment of vaccines in response to out-breaks.
- The need for ways to accelerate vaccine FDA licensure without compromising product safety, for example use of the proposed animal efficacy rule for products that are either not feasible or ethical for human efficacy trials.
VACCINES FOR THREATENING AGENTS: ENSURING THE AVAILABILITY OF COUNTERMEASURES FOR BIOTERRORISM *
Affiliations.
Recent events have brought the subject of vaccines as a defense against bioterrorism into very sharp focus. We have been forced to take action in an area that, for the civilian sector, had previously been largely an academic debate and planning exercise with inadequate definitive action. We have changed from a nation of skeptics concerning the threat of bioterrorism to a nation of believers. Several government agencies are working at unprecedented speed to acquire the needed vaccines and put the correct policies into place for utilization.
However, the use of vaccines for defense against bioterrorism presents multiple challenges that are quite different from the traditional public health use of vaccines for protection against endemic or epidemic diseases. The issues are also quite different from those faced by the armed forces. The appropriate use of vaccines as a defense against bioterrorism presents major challenges in public policy development as well as public education. The ongoing public debates in the media highlight the complexity of the issues and reveal the widespread lack of understanding of the limitations of the current vaccines, especially vaccinia vaccine. For example, there is a call for widespread vaccination against smallpox but, in contrast, there is much misinformation and inappropriate fear about the effects of anthrax vaccine.
Some of the challenges involved with developing vaccine policies for defense against bioterrorism lie in the uncertainty of the threats. In contrast, policies for the use of vaccines against naturally-occurring disease threats are based on a wealth of historical and current epidemiologic information about disease burden and potential. Additionally, there is extensive data available on the safety of widely used vaccines that can be used to confidently assess risk benefit and cost effectiveness. In the case of agents of bioterrorism, however, risk assessment is much more difficult. The great difficulty in obtaining timely and reliable intelligence on the threat of biologic terrorism is a major part of the problem. Critical policy decisions—such as which vaccines will be needed, how large the stockpiles should be, and how the vaccine should be used—are greatly influenced by perceptions of threat. The very nature of terrorism produces a high level of uncertainty about what to expect and prepare for, and there is a wide and varying spectrum of perceived threats.
Obtaining the vaccines that are needed to protect our military and civilian populations depends entirely on effective government action. The potential market has been too small, at least up to the present time, to encourage the vaccine industry to make the large investments needed in research, development and manufacturing facilities. This has changed dramatically in the past two months. Nevertheless, the current situation is a result of past misjudgments, which resulted in insufficient government investment in vaccine research and development, and manufacturing capacity. There is an urgent need for rapid progress in R&D, manufacturing, and licensing processes, all of which are painfully slow processes when done by the usual methodologies.
Vaccines have varying usefulness in defense against bioterrorism. At the top of the list is the need for smallpox vaccine to prevent an outbreak from becoming a catastrophic global pandemic. Both smallpox and anthrax vaccines would be very useful in the medical management of exposed individuals, if the vaccines were readily available and placed in geographic proximity to multiple centers for distribution. Less important to the civilian populations are vaccines against bacterial agents that can be managed with antibiotics and viral agents which, at least for the present, seem to be lesser threats. These include plague, tularemia, hemorrhagic fever viruses, alphavirus encephalidities, Rift Valley fever, and others. However, several of these vaccines should be available for both civilian and military use. A government-owned production facility may be the best means for meeting the needs of these lower priority vaccines which will probably, at least initially, be made in much smaller quantities than smallpox and anthrax vaccines.
Smallpox Vaccine
The acquisition of a smallpox vaccine stockpile for civilian use started in 1999, with an Acambis contract for 40 million doses which now has been increased to 54 million doses. The seed virus was developed by cloning a New York City Board of Health strain derived from Wyeth Dry Vax. Animal model studies indicate that this strain appears to be somewhat less neurovirulent than the parent virus. The clinical development plan is aggressive; the phase I clinical trial should occur, as planned, in the latter part of January 2002. A very rapid procurement action has been in progress over the past weeks. The goal is to stockpile enough smallpox vaccine to protect the entire nation within the year. The response from the vaccine industry has been very heartening and has provided excellent options for utilizing existing manufacturing capacity to meet current requirements. Every effort will be made by CDC, FDA and NIH to assure that these contractors succeed to meet goals, time lines, and regulatory requirements. This will require truly unprecedented coordination and responsiveness by both the manufacturers and the various agencies.
Although the first step in building the smallpox vaccine stockpile is to ensure that vaccine manufacturing is underway, there are several other immediate issues that need to be addressed:
- Vaccination policy issues continue to be controversial. The CDC recently sent out a draft smallpox response plan to the states for comment. The plan calls for primary reliance on ring vaccination—the traditional method—to control an outbreak. The CDC has vaccinated 140 staff members who are most likely to be involved in investigating an outbreak, but no further vaccination with potential responders or health care providers is planned at this time. Laboratory personnel working with pox viruses will, of course, continue to be vaccinated.
- There is a need for more vaccine immune globulin (VIG) or VIG substitute to deal with the consequences of vaccination in immunosuppressed or other high risk subsets of the population. An interagency working group is currently exploring options for expanding the VIG supply.
- There is a need to develop a safer vaccine for use in immunosuppressed individuals, pregnant women, and other individuals for which the current vaccine is contraindicated. This will not only be a challenging research and development problem but also a challenging regulatory problem due to the difficulties in proving efficacy.
Anthrax Vaccine
The current licensed U.S. anthrax vaccine is a filtrate of culture media that contains a high level of PA (protective antigen) absorbed to alum; it probably contains small amounts of the other factors as well. An ongoing study at CDC is testing immunization schedules that involve fewer than the currently recommended six doses for this vaccine. Conventional wisdom has it that the live attenuated vaccines used in Russia and China are too reactogenic to be licensed in the United States. Israeli scientists have published reports on animal studies of experimental vaccines engineered to over-express recombinant protective antigen, but no clinical data are available.
The problems that the manufacturer has had with meeting regulatory criteria have limited the U.S. supply. A small amount of anthrax vaccine has been made available to DHHS by DoD, but that amount is far below what will be needed. There is an urgent need for a sufficient supply of anthrax vaccine for vaccinating high risk populations and for use as post-exposure vaccination in conjunction with antibiotics.
There are several immediate issues that need to be addressed. The production method for current licensed vaccine must be scaled up. Several commercial firms have made informal proposals to do this. However, this is a high risk option because of the inherent variability of the manufacturing process and the high risk of failure when scaling up so rapidly to such a high volume. There needs to be more serious consideration of the applications of the various platform technologies—such as plasmid DNA, viral vectors, and a variety of other delivery technologies—that are being developed within the biotech industry.
Finally, we need to accelerate development of a second generation vaccine. The time to availability could be shortened by overlapping large scale production with clinical trials. It has been suggested that we might have a stockpile of IND recombinant protective antigen (PA) vaccine within 18 months. This may be an achievable goal if all involved interests work in an effective, coordinated manner. A recombinant PA vaccine produced in E. coli will likely be the first to enter a phase I trial.
In order to address this issue of a second generation vaccine, the National Institute of Allergy and Infectious Diseases has put together a team with contractor help. Efforts are underway to gather all available information on ongoing or planned development efforts for a second generation anthrax vaccine, and compile the information in a systematic fashion and convene several advisors to review the resulting data, findings, and policy options. This may involve a major research and development contract program similar to what exists for smallpox vaccine and which will hopefully build on the work that has been done by DoD and DoD-DHHS collaboration. It will hopefully involve some new players as well, including the large vaccine manufacturers. Although it is difficult to predict which particular options will receive aggressive support, there is nonetheless a system now in place that will hopefully pave the way for pursuing an effective strategy in a reasonable period of time. The speed at which a second generation anthrax vaccine is developed will depend on both the underlying science and the responsiveness of the vaccine industry to national needs.
THE DEPARTMENT OF DEFENSE AND THE DEVELOPMENT AND PROCUREMENT OF VACCINES AGAINST DANGEROUS PATHOGENS: A ROLE IN THE MILITARY AND CIVILIAN SECTOR? *
Introduction.
In October 2001, the threat of bioterrorism became a reality. In support of this Forum's efforts to identify the obstacles to preparing an optimal response to bioterrorism—particularly as it relates to the complexities of interaction between private industry, research and public health agencies, regulatory agencies, policymakers, academic researchers, and the public—this paper will highlight emerging opportunities for more effective collaboration as well as scientific and programmatic needs for responding to bioterrorism. The focus of this paper is on the potential opportunities and issues related to Department of Defense (DoD) support for the research, development, and production of biological defense vaccines for the military and civilian populations to protect against bioterrorist threats. This paper will address the following topics:
- Current medical biological defense research and development efforts;
- Current biological defense vaccine capabilities;
- Proposed national biological defense vaccine production facility; and,
- Issues related to the use of biological defense vaccines.
In accordance with Congressional direction, DoD established a Joint Service Chemical and Biological Defense Program in 1994. The vision of the program is to ensure U.S. military personnel are the best equipped and best prepared force in the world for operating in future battlespaces that may feature chemical or biological contamination. The capabilities being developed for the military may have applicability to protection of civilians, especially as the military mission may increasingly support homeland security. Vaccines to protect against biological agents provide one critical capability to protect against the threat.
Medical Biological Defense Research and Development Efforts
The primary research program for the development of biological defense vaccines to protect U.S. forces is the Medical Biological Defense Research Program (MBDRP). In developing countermeasures to biological agents, the MBDRP uses a technical approach that focuses on four areas:
- Identify mechanisms involved in disease process;
- Develop and evaluate products (vaccines or drugs) to prevent or counter effects of toxins, bacteria, viruses, and genetically engineered threats;
- Develop methods to measure effectiveness of countermeasures in animal models that predict human response; and,
- Develop diagnostic systems and reagents
Biological defense vaccines are being developed to counter viruses, toxins, bacteria, and genetically engineered biological threat agents. Research activities start with basic research activities and proceed through the following steps, as research demonstrates successful candidates: (1) construction of the infectious clone, (2) identification of attenuating mutations, (3) construction of vaccine candidates, (4) testing in rodent models, (5) testing in non-human primates, (6) final selection, and (7) formulation. The formulated production may then become a candidate for an Investigational New Drug (IND) application for transition to advanced development and clinical trials, then ultimately licensed production.
An example of a product being developed within the MBDRP is the Next Generation Anthrax Vaccine. In cooperation with the National Institutes of Health, the next generation vaccine will provide greater or equal protection, require fewer doses to produce immunity, and have fewer adverse effects than the current anthrax vaccine. The reduced number of doses would provide greater flexibility to military forces by reducing the time constraint for developing immunity, hence accelerating the time for fielding a protected force. The next generation vaccine is based on recombinant protective antigen (rPA), which binds to the lethal factor (LF) and edema factor (EF) of B. anthracis . The recombinant production technology would eliminate need for spore-forming anthrax, and hence the need for a dedicated production facility. Overall, the next generation anthrax vaccine would decrease production cost, allow a greater range of potential vaccine production facilities, and potentially allow for streamlining of the regulatory approval process.
Another example of a product being developed within the MBDRP is Multiagent Vaccines (MAV) for Biological Warfare (BW) Threat Agents. The MAV project is a proof-of-principle effort to construct a vaccine or vaccine delivery approach that could concurrently immunize an individual against a range of BW threats. Bioengineered and recombinant vaccine technologies will be exploited to achieve vaccines that are directed against multiple agents, yet use the same basic construct for all of the agents. The MAV would be analogous to commercial vaccines (e.g., measles-mumps-rubella) but would exploit new approaches—naked DNA vaccines and replicon vaccines. The MAV would result in a reduced number of doses and thus provide greater flexibility to military forces by reducing the time constraint for developing immunity, hence accelerating the time for fielding a protected force. The MAV also could decrease production cost, allow for greater range of potential vaccine production facilities, and potentially allow for streamlining of the regulatory approval process.
Current Biological Defense Vaccine Capabilities
Joint vaccine acquisition program (jvap).
In order to enable the transition of candidate biological defense vaccines developed under the MBDRP or from other sources, a Prime Systems Contract was awarded in November 1997 to DynPort Vaccine Production Corporation, LLC. The JVAP was established for the purpose of developing, testing, and Food and Drug Administration (FDA) licensure of vaccine candidates, and production and storage of vaccine stockpiles. A major objective of the program is to establish a viable industrial base for vaccine production. The next generation anthrax vaccine (rPA) is one of several vaccines being investigated for development by the JVAP. Other vaccines in advanced development include smallpox, pentavalent Botulinum Toxoid, and tularemia. The Prime Systems Contract also provides options for other biological defense vaccines. Currently, all vaccines in the JVAP are in the development phase.
Anthrax Vaccine Adsorbed (AVA) and the Anthrax Vaccine Immunization Program (AVIP)
The only vaccine currently licensed for use in the United States to protect against anthrax is AVA. AVA is cell-free filtrate, produced by an avirulent strain of Bacillus anthracis . It is manufactured by BioPort Corporation in Lansing, Michigan and procured under a separate contract. It was licensed by the FDA in 1970. Six doses of the vaccine are required for full immunity, including doses at 0, 2, and 4 weeks, 6, 12, and 18 months, followed by an annual booster.
On December 15, 1997, the Secretary of Defense approved the decision to vaccinate all of the U.S. armed forces against anthrax, contingent on the successful completion of four conditions, which were met: supplemental testing of the vaccine; tracking of immunizations; approved operational and communications plans; and review of health and medical aspects of the program by an independent expert. Implementation is determined in accordance with DoD Directive 6205.3, “DoD Immunization Program for Biological Warfare Defense,” November 26, 1993, with complete implementation of the plan contingent upon adequate supply of the licensed vaccine
On May 28, 1998, the Secretary of Defense directed vaccination of the total force. Implementation of this directive was administered by the AVIP. As of May 29, 2001, more than two million doses were administered to more than 500,000 military personnel, with at least 70,000 completing the full six-shot regimen. Since then, there has been only a few who have received vaccinations. As outlined in a June 8, 2001 memorandum, the Secretary of the Army ordered a slowdown in immunization to accommodate delays in release of vaccine pending FDA approval. Implementation of the vaccination continues to designated special mission units, to vaccine manufacturing and DoD personnel conducting anthrax research, and others conducting Congressionally mandated anthrax vaccine research. Detailed information on the status of the AVIP is available at www.anthrax.osd.mil .
What Does Producing a Vaccine Mean?
With no vaccines currently in production under the JVAP and AVA as the only currently available FDA licensed vaccine for protection against BW threats, DoD is evaluating other mechanisms to increase and sustain vaccine production. In order to identify the status of vaccines, it is important to understand the major phases of research, development, and production through which they must proceed. Within different phases of vaccine development and production, there will be varying levels of production risk and overall risk. There are three major phases in the development and production of new vaccines—science and technology base, development and licensure, and licensed production. Following is a summary comparing different activities within each phase.
Production Approach
Within the science and technology phase, production is focused on small quantities and relies on bench top methods, which may include many different approaches, including new state-of-the-art experimental approaches. When a candidate product transitions to the next phase, a best approach is selected (or in some cases two or three promising approaches) and tested for scale up for full scale production. Following licensure, production proceeds at full scale and relies on a single, fixed method. Changes in the method typically require further testing and require approval by the FDA.
Vaccine Recipients
Perhaps the most obvious difference among the phases are the numbers and types of vaccine recipients and the purposes for which they receive the vaccine. Within the science and technology phase, recipients are primarily laboratory animals and include hundreds of animals. The primary purpose for using these recipients is to demonstrate the potential effectiveness of a vaccine candidate, that is proof-of-principle testing. During the development and licensure phase, vaccine recipients are humans, who participate in clinical trials. All recipients are volunteers, who participate in clinical trials that comply with FDA regulations. The focus of these investigations is to determine the safety and efficacy of a vaccine as well as to optimize dosing and scheduling. The final phase is production and includes providing a licensed vaccine to all individuals who may be at risk, in accordance with the FDA license and based on quantities available, for the purpose of providing protection against potential threats. The effected populations could be on the order of millions of individuals.
Production Risk
Production risk during the science and technology phase is moderate since only small quantities can be produced yet only small quantities are needed. Risk is minimized since FDA approval of the product is not required. During the development and licensure phase, production risk is usually high because of the risks involved in scaling up pilot lot product to full scale production. Overall risk is also high because of reliance on and surrogate models or biomarkers to determine efficacy, since law prohibits exposure of humans to chemical or biological agents.
Overall Risk
Overall risk for production of biological defense vaccines will vary depending on the type of vaccine being produced and the policy implemented for immunization. For example, use of a live vaccine (e.g., vaccinia live vaccine) poses risk that inoculated individual may be giving off live vaccinia viruses until scarification has occurred (2–5 days), hence potentially exposing unprotected individuals. Another risk is that low rates of adverse effects may become more apparent in a large scale immunization program than had occurred during testing. For example, if 1,000 people are tested in clinical trials and only one had a serious adverse reaction, there may be hundreds of reactions if the total military force is vaccinated.
Biological Defense Vaccine Development and Production Issues
One of the major factors limiting the availability of biological defense vaccines is the limited interest from the pharmaceutical industry in supporting the production of these vaccines. In contrast to vaccines to support public health needs (e.g., childhood diseases, influenza), most vaccine needs are fulfilled by the private sector. However, the private sector has some challenges in fulfilling public health vaccine needs. The vaccine production industrial base is nearly at full capacity to meet public health priorities. This will pose a challenge for the production of biological defense vaccines if production of biological defense vaccines results in the deferral of production of public health vaccines. Biological defense vaccines are considered specialty biologics and interest is primarily centered on a few small to mid-sized companies. Industry interest is limited in part because of requirements to conduct large, complicated clinical studies to demonstrate safety, immunogenicity, and efficacy (where possible).
Another major factor effecting the timely availability of biological defense vaccines are issues related to compliance with Chapter 21 of the Code of Federal Regulations (21 CFR), Food and Drug Administration (FDA). The specific issue relates to the ability to determine the clinical efficacy of biological defense vaccines. 21 CFR requires that for efficacy to be established, vaccines must be tested in informed, volunteer human subject who are exposed to the condition against which the vaccine is intended to protect. However, legal and ethical constraints prohibit exposing human subjects to biological agents. This constraint plus limited availability of human data for most vaccines mean that under current regulations, biological defense vaccine efficacy cannot be established. In order to address this constraint, FDA published a proposed rule on October 5, 1999 entitled, “New Drug and Biological Products; Evidence Needed to Demonstrate Efficacy of New Drugs for Use Against Lethal or Permanently Disabling Toxic Substances When Efficacy Studies in Humans Ethically Cannot Be Conducted; Proposed Rule.” (FDA rules are available at http://www.fda.gov/cber/rules.htm .) The proposed rule is expected to be finalized during 2002. Under this rule, efficacy may be determined based on data from clinical testing on animals (using at least two different species with preference that non-human primates be one of the species.) Animal data would serve as a surrogate for human data, but there would need to be significant data demonstrating that the effects in animals is related to effects in humans. Without the ability to license vaccines based on surrogate test data, biological defense vaccines would remain as investigational new drugs, which would continue to limit availability.
Proposed National Biological Defense Vaccine Production Facility
Following years of research, development, and efforts to produce biological defense vaccines in sufficient quantities to meet DoD needs, a different approach is currently being planned. In July 2001, DoD submitted a report to Congress detailing biological defense vaccine efforts within DoD. Known as the “Top Report”—because it provides the results of an independent expert panel chaired by Franklin Top, M.D.—this report summarized key shortcomings of current biological defense vaccine acquisition efforts. The report made the following findings and recommendations:
- The scope and complexity of the DoD biological warfare defense requirements are too great for either the DoD or the pharmaceutical industry to accomplish alone,
- The panel recommended a combined integrated approach whereupon DoD would work closely with the vaccine industry and national scientific base, and
- The panel recommended the construction of a government-owned, contractor operated (GOCO) vaccine production facility, which would include production capacity for up to eight vaccines over the next 7–12 years and would cost an estimated $2.4–$3.2 billion over that time.
The report recognized that in order for the GOCO to be successful, it would require long-term government commitment, increased resources, innovative DoD business and program management practices, and effective participation by established pharmaceutical industry leaders in vaccine discovery, licensure, and manufacturing.
The design concept for a GOCO biological defense vaccine production facility would accommodate three bulk vaccine production suites, each with different processes: spore-forming bacteria (for which FDA requires separate facilities), microbial fermentation, and tissue culture (viral vaccines). A modular design would allow flexible and expandable manufacturing capacity for production of DoD-cntical vaccines that are intended for force health protection.
The scale of the facility will be determined in part by the quantity of vaccines to be produced. The assumptions for the production capacity requirement are categorized into three tiers. Tier 1 is the baseline requirement and reflects current production requirements, which is the same as current requirements for the JVAP and AVIP. This tier includes sufficient anthrax vaccine for the entire force (approximately 2.4 million doses). It additionally would require 300,000 Troop Equivalent Doses (TEDs) for other biological defense vaccines. (Troop equivalent dose is defined as the number of vaccine administrations to reach full immunity. Boosters are not included.) Tier 2 would require three million TEDs (2.4 million for U.S. forces + 0.6 million for Commanders Reserve) of each vaccine to be produced to allow for total force protection plus sufficient quantities to support annual requirements due to personnel turnover. This requirement was the basis for the initial GOCO cost estimate. Tier 3 would require approximately 300 million TEDs of each vaccine to support civilian protection for the entire U.S. population.
In order to define the requirements for vaccine production and to ensure that it addresses national, and not just DoD needs, an interagency advisory group has been established. Interagency participation has been led by DoD and the Department of Health and Human Services, with participation from several organizations (including the Office of Homeland Security) to ensure a broad perspective. Federal participation is essential since biological defense vaccine needs are not being met by private industry. No individual department has the sufficient, full-spectrum capability and capacity to support vaccine needs. A national vaccine authority may be essential to ensure interagency needs are addressed not only in the planning phase but also in implementation. The details of the national vaccine authority are being developed, though it is not likely to be established as a new agency.
Issues Related to the Use Of Biological Defense Vaccines
Why vaccinate vaccine use risk management decisions.
BW agents pose high risk to military forces and operations, and at least ten countries are pursuing offensive BW programs. Vaccines are the lowest risk, most effective form of protection against BW threats. Vaccines are more effective and have fewer adverse effects than antibiotics or other treatments following exposure. While masks may provide highly effective protection, they may impede performance and must be worn to provide protection. Vaccines enable force protection by providing continuous, long-lasting protection. In addition, there are currently no real-time BW detection systems available. While there are systems that provide the ability to detection respirable aerosols in near real-time, the best available systems today take 15–45 minutes to identify a specific BW agent.
Vaccines are unusual among medical products in that they are given to healthy people to keep them healthy. Table 3-1 shows several of the vaccines commonly given to protect against infectious diseases and contrasts them with the limited number of biological defense vaccines currently available. Biological agents that may be used as weapons may be naturally occurring but have a very low incidence of natural occurrence (at least in the United States.)
Selected infectious diseases vaccines and biological defense vaccines.
The risk assessment for using biological defense vaccines is different from naturally occurring infectious diseases ( Grabenstein and Wilson 1999 ). Because to vaccinate is based on potential risk of disease outbreak rather than actual incidences. Consequently, a proper risk assessment for biological defense vaccines should not be a trade-off assessment between the actual adverse effects of a vaccine vs. the actual adverse effects of the disease, but the actual adverse effects of a vaccine vs. the potential adverse effects of the disease.
The policies on the use of biological defense vaccines will affect biological defense vaccine manufacturing. The two basic options for immunization are stockpiling vaccines in anticipation of a specific contingency or routine use immunization to ensure continued general readiness. If vaccines are stockpiled, manufacturing must address issues related to maintaining the stockpile as a result of the limited shelf life of some vaccines. Additionally, if vaccines are produced in bulk, once the required quantities are produced, manufacturers must ensure that the facilities remain capable of retaining an FDA facility license when production is not ongoing.
The assessment of potential and actual effects may effect product development. For example, as polio has been significantly reduced as a result of extensive vaccination, the Centers for Disease Control have recommended use of the inactivated polio vaccine (IPV) rather than the oral polio vaccine (OPV). While OPV has greater efficacy, it is also linked with rare occurrences of vaccine-associated paralysis. As cases of polio have been virtually eliminated in the United States, the risk of rare occurrences of adverse effects of the vaccine has exceeded the risk of the occurrences and effects of the disease.
If biological defense vaccines are produced and planned for use—especially among civilians populations—vaccine development criteria may place greater emphasis on vaccine safety than on vaccine effectiveness. Risk assessments may be complicated by the fact that the limited industrial base capacity for biological defense vaccine production will likely result in only one vaccine being available for military and civilian use.
There are other key differences between the military and civilian populations that make risk assessment difficult. One factor is that biological defense vaccines made for the military population are intended for use only in healthy adults. By contrast, the general population will also include significant subgroups for which vaccine safety, efficacy, or dosing information may not be fully understood, including pediatrics, geriatrics, pregnant women, and immune-compromised individuals. Currently there is no policy in place to immunize the civilian population absent a naturally occurring threat. If a licensed biological defense vaccine were available for use by the general population, an immunization policy for civilian use would be needed to address several issues before immunization could begin. Some of the issues that would need to be addressed are, for example, who would be vaccinated—the entire population, or a subgroup? Which subgroup(s)? Those living in specific regions? First responders? If symptoms of biological agent do not appear, would that be interpreted as the absence of a threat or the effectiveness of the defense? Paradoxically, would the demand for the vaccine diminish as the apparent threat also diminished? Civilians may also have greater concerns about the long term safety effects as a result of vaccine use. Additionally, there may be concerns regarding the unknown safety of the use of biological defense vaccines when interacting with other medical products. While there is no adequate basis to assess safety, there is no basis for extraordinary concern ( Institute of Medicine, 1996 ).
Conclusions
The Department of Defense may bring valuable assets to bear to counter the use of biological agents by terrorists. Currently, the DoD mission is focused on responding to threats to the military. Because of DoD's experience in defending against biological threats, DoD will continue to play a role in addressing the threat to the civilian population as well. DoD will continue to work with other agencies, including the new Director of Homeland Security, to determine what role it will play in homeland security, which will be defined in The Federal Response Plan, presidential directives, and other sources.
The availability of vaccine to protect against anthrax and other biological agents is based on several factors. One key factor is sustained resources to transition products from the science and technology base to advanced development. Resources include not only adequate funding, but also trained personnel, which is a critical factor since the biotechnology and pharmaceutical industry as a whole is facing shortages of skilled personnel. A second factor limiting the availability of biological defense vaccines is that they are similar to orphan drugs. There is no commercial incentive for manufacturers to produce vaccines. Federal investment may be required to retain the services and capabilities of the biotechnology and pharmaceutical industry.
While the availability of vaccines is critical, the decisions of whether to vaccinate will remain equally important. Vaccination decisions will continue to have greater physiological consequences than non-medical measures to protection against the threat (e.g., whether to wear masks). The decision will need to weigh the risk of actual low rates of adverse effects against the potential for protecting against catastrophic effects. In making these decisions based on risk, communicating the risk decision will be at least as important as risk assessment. Failure to have a coordinated public policy decision on vaccination support for civilians may result in individuals self-prescribing treatments or failing to comply with recommended guidelines.
APPLICATIONS OF MODERN TECHNOLOGY TO EMERGING INFECTIONS AND DISEASE DEVELOPMENT: A CASE STUDY OF EBOLA VIRUS *
In recent years, increasing attention has been focused on the Ebola virus as a potential public health problem, either from natural or deliberate outbreaks. Like the genetically related Marburg virus, Ebola is a filovirus that causes highly lethal hemorrhagic fever in humans and primates. Infection rapidly progresses from flu-like symptoms to hemorrhage, fever, hypotensive shock, and eventually, in about 50–90% of cases, death ( Peters et al., 1996 ; Peters and Khan, 1999 ). The molecular mechanisms underlying the pathogenicity of the Ebola virus are not well understood, in part because it has emerged only relatively recently (for reviews see Balter, 2000 ; Colebunders and Borchert, 2000 ). There was a series of outbreaks in central Africa in the mid-1970s and again in the 1990s (i.e., the Ivory Coast in 1994, Gabon in 1994–1996, Zaire in 1995, Gulu, Uganda in 2000 and presently in Gabon and the Republic of Congo). Ebola virus infection has appeared once in the United States, in Reston, Virginia. The Reston strain is not pathogenic in humans, and the outbreak was fortunately restricted to non-human primates.
One of the reasons that Ebola is highly lethal is that this virus replicates at an overwhelming rate ( Sanchez et al., 1996a ). Thousands of Ebola virus particles per host cell can completely envelop the cell and take over its entire protein synthetic machinery. We have only recently begun to understand the molecular mechanisms underlying this phenomenon. Although we have a descriptive understanding of the cytopathic effects of viral replication, we lack a clear understanding of how these various changes in cell structure and viability occur. Elucidating these details will be critical for developing vaccines and other antiviral therapies.
Aside from the obvious immediate health threat that would be posed if it were introduced into the population, Ebola virus represents a useful paradigm for dissecting the molecular genetics of a virus. Most of what is known about Ebola pathogenesis is derived from genetic studies of the virus. Although Ebola is very similar to the genetically related Marburg virus, it differs in at least one important respect. The gene that encodes the viral glycoprotein in Ebola generates two gene products, whereas in Marburg, this gene encodes a single protein (Sanchez et al., 1996). One of the gene products is secreted as a soluble 50 to 70 kDa glycoprotein, whereas the other is a full-length 120 to 150 kDa glycoprotein that inserts into the viral membrane ( Volchkov et al., 1995 ; Sanchez et al., 1996). The secreted form was originally believed to serve as an immunological decoy for the full-length glycoprotein, allowing the full-length glycoprotein to attach to the target cell. However, more recent evidence now suggests that this hypothesis is unlikely. Instead, the secreted form appears to inhibit early steps in neutrophil activation and thereby inhibit the host inflammatory response to the virus ( Yang et al., 1998 ). The secreted glycoproteins have been shown to bind quite well to neutrophils, but bind poorly to endothelial cells ( Yang et al., 1998 ). In contrast, the full-length glycoprotein interacts with endothelial cells but binds poorly to neutrophils ( Yang et al., 1998 ). This glycoprotein enables the Ebola virus to recognize and introduce its viral contents into the endothelial cell lining of the blood vessels, as well as monocytes/macrophages, thereby resulting in the cellular damage that is associated with the devastating symptoms of Ebola infection.
Antiviral Targets
Detailed analyses of the mechanisms of viral entry, replication, and cell damage have identified the Ebola glycoprotein 2 (GP2) as a potential antiviral target. In particular, there is a region in the GP2 ectodomain of Ebola virus that forms a coiled coil, or hairpin-like structure similar to what exists in the human immunodeficiency virus (HIV), influenza, respiratory syncytial virus, and a variety of other viruses ( Weissenhorn et al., 1998a , 1998b ; Malashkevich et al., 1999 ). This coiled-coil region contributes to membrane fusion by undergoing conformational changes after the glycoprotein binds to the membrane receptor ( Weissenhorn et al., 1998b ; Watanabe et al., 2000 ). The fact that this structure is conserved in a number of different viruses suggests that it may represent a potential target for antiviral therapy. In fact, a peptide product directed at the analogous structure in HIV has potent antiviral effects and is currently being developed for the clinical treatment of AIDS. This or similar peptides could be useful against many other viruses as well, including Ebola.
Not only does the transmembrane glycoprotein direct the Ebola virus into specific cells, but the glycoprotein itself is also highly toxic to cells. For example, when full-length Ebola glycoprotein is overexpressed in cultured renal epithelial cells, it inserts into the membrane and causes morphological changes and detachment from culture dishes ( Yang et al., 2000 ). This finding suggests that there is a genetic determinant in the glycoprotein that mediates its toxicity and, therefore, might represent another potential target for antiviral therapies. Mapping studies identified a serine-threonine-rich, mucin-like core domain region of the glycoprotein that is required for cytotoxicity in human endothelial cells ( Yang et al., 2000 ). When the mucin-like region of the glycoprotein was deleted, its cytotoxicity was abolished, but protein expression and function remained unchanged ( Yang et al., 2000 ). Every possible open reading frame in the Ebola virus genome has been tested for toxicity, except for the polymerase region. To date, only the glycoprotein has been shown to induce toxic cytopathic changes. However, a better understanding of the detailed molecular mechanism of virus assembly may eventually provide insight into other potential antiviral targets as well.
Vaccine Development
Not only does the glycoprotein play an important role in toxicity, increasing evidence suggests that it also plays an important role in the pathogenesis of Ebola infection. Infection of cultured cells with adenoviral vectors encoding the glycoprotein causes considerable cellular damage that correlates with toxicity. However, overexpression of a glycoprotein that is unable to insert into the cell membrane is not cytotoxic. In fact, injecting adenoviral vectors, or DNA forms of these vectors, into mice, rabbits, and primates actually protects the animals from disease by inducing an effective vaccine response. No human vaccine against Ebola is currently available. However, studies in animals suggest that DNA vaccines, together with replication-defective adenoviral vectors, may be particularly promising. In the DNA vaccination platform, a plasmid expression vector is injected into muscle, thereby enabling muscle to synthesize large quantities of proteins that stimulate the immune system to generate an effective immune response. DNA vaccination technology could greatly simplify the vaccination production process that would otherwise rely on very large-scale plants for making these complex and highly purified proteins. However, although current DNA vaccines work well in rodents, they are not as effective in non-human primates and are even less robust in humans. Thus, one of the important challenges for developing an effective DNA vaccination platform technology is to improve immune responses in non-human primates and humans.
The first successful studies of a DNA vaccine for Ebola virus were carried out in guinea pigs (Xu et al., 1999). Animals that were immunized with sufficient levels of Ebola virus glycoprotein to induce a high-titer antibody response survived infection. Guinea pigs with intermediate levels of titers exhibited an intermediate chance of survival. In contrast, none of the control animals, immunized with vector alone, survived Ebola infection (Xu et al., 1999). “Prime-boost” strategies combine DNA immunization and boosting with adenoviral vectors that encode viral proteins to specifically target dendritic cells. Such DNA vector-viral vector combinations can be very potent. Animals are first immunized with a DNA vector, and typically develop titers ranging from 1:1,500 to about 1:3,500. Following the adenovirus boost, antibody titers increase dramatically, ranging from 1:50,000 to 1:100,000. This far exceeds the minimum threshold that is considered to be necessary for an effective immune response in primates. A modified prime-boost strategy was recently used to immunize cynomolgus macaques against several strains of the Ebola glycoprotein ( Sullivan et al., 2000 ). Several months later, animals were boosted with recombinant adenovirus expressing the Ebola (Zaire) glycoprotein. Control animals received empty vectors consisting of plasmid DNA and ADV-DE1 recombinant adenovirus in a parallel injection regimen. When animals were subsequently challenged with a lethal dose of the Zaire subtype of Ebola virus, all control animals (6 out of 6) exhibited rapid increases in their viral antigen levels and succumbed to infection within seven days. In striking contrast, all animals immunized with the combination DNA-adenovirus vaccine survived Ebola virus challenge (4 out of 4). The level of antibody production and the cellular proliferative response were closely correlated with immunoprotection.
It is of interest to note that vaccines are not only clinically useful, but they can also serve an important function as deterrents against bioweapons. It is much more difficult to engineer vaccine resistance than drug resistance in an organism. Having well-defined, publicly known, and effective vaccines is a critical preventive, or deterrent, strategy. Another benefit of a successful vaccine is that it opens the way for developing novel immunotherapies. In the case of Ebola virus, for example, hyperimmune serum from animals that are protected from the disease is currently being examined, to determine if it can be used during the course of infection as a possible post-exposure therapy.
Role of Genomics in Vaccine Development and Biodefense
Genomic approaches hold enormous potential for vaccine development, and these possibilities are only just beginning to be explored. For example:
- Analysis of global gene expression patterns can facilitate the early identification of both environmental and disease-associated pathogens.
- Gene expression patterns can be used to identify specific genetic susceptibility and resistance markers.
- Biomarkers for vaccine efficacy could be incorporated into the experimental design of efficacy trials, which could then accelerate approval and licensure processes.
- High throughput technology can be used to improve vaccine design, by allowing researchers to readily monitor how specific structural changes in the vaccine affect the cellular response to immunization.
It is possible that enough information will eventually be available and implemented within the technology that simply knowing the sequence of a particular open reading frame will be sufficient to understand how to generate an effective vaccine. Such technology would be useful not only as a defense measure against bioterroism, but also for the prevention or treatment of naturally occurring outbreaks, such as influenza. The influenza virus constantly mutates, but if genetic information could be acquired quickly enough, it may become possible to develop more effective countermeasures.
In conclusion, the process of vaccine development must evolve to become more responsive to the changing needs and emerging outbreaks of society today. In other words, more agile vaccines are needed. Agility includes the ability to rapidly deploy vaccines in the event of an outbreak; to accelerate immunization regimens so that such an outbreak could be effectively managed; and, finally, new technology must be applied to develop better vaccines and to accelerate the development process.
MEETING THE REGULATORY AND PRODUCT DEVELOPMENT CHALLENGES FOR VACCINES AND OTHER BIOLOGICS TO ADDRESS TERRORISM *
The FDA plays an important role in multiple stages of the product development process, from initial clinical studies through licensure, manufacturing and post-marketing studies which may be used to further evaluate safety and effectiveness. For these reasons, FDA is committed to working together with the scientific and clinical communities and with industry and the public to fulfill its regulatory and public health role in facilitating the development of biodefense biologics and therapeutics. Recent and ongoing FDA biodefense-related activities include, for example, meeting with sponsors and sister agencies and departments to encourage interest in developing safe and effective new products needed for public health biodefense, performing research that ultimately facilitates the development of these products; and providing intensive and early interactions with product sponsors to speed their availability.
As with any medical product, bioterrorism products need to be regulated to ensure consistent and objective protection of the public safety. While there is currently a sense of emergency and a set of urgent needs to address, the desire for rapid and innovative responses must not be allowed to compromise the objective assessment of safety and effectiveness. Thus we need a regulatory agency that can step back and provide a more objective perspective. If and when things go wrong in the wake of decision(s) made in a time of crisis, few people will remember the crisis and that the decision was in fact made with the best intentions. The public expects safe and effective products, and safety expectations are especially high for vaccines administered to healthy individuals. Maintaining public confidence in vaccines and medical products, in general, is critical to maintaining overall confidence in our nation's public health programs and leadership in matters extending far beyond bioterrorism. For these reasons, even in difficult times, we must continue to make and communicate clearly the best possible scientific and public health decisions about product development, licensure, availability and use.
Furthermore, bioterrorism is a moving target, not a single disease of predictable epidemiology, and all potential product uses may not be anticipated. This complicates many decisions about product use. For example, a vaccine, such as the licensed anthrax vaccine, which may have been originally studied and used in a limited population effectively and without major safety concerns may raise more significant public concerns about uncommon adverse events, whether coincidental or due to the vaccine, if and when it is administered for similar reasons to hundreds of thousands of people or when unanticipatedly used for post-exposure prophylaxis.
There are several factors that account for why we do not have an adequate supply of vaccines for bioterrorism defense:
Financial Disincentives
- Uncertain markets, especially for potentially more limited use products such as a tularemia or plague vaccine.
- Uncertain longevity of the needs, markets and of resources; short attention spans in government budgeting.
- The fact that vaccines are complex biological products that carry a high risk of uncertainty, unpredictability of success, and financial loss.
- The rigorous safety requirements and low public tolerance of risk—in part because they are often administered to healthy people as a preventative measure—and associated costs of developing biologics.
- The fact that preventive measures are generally undervalued, both perceptually and financially. Vaccines are often expected to be sold for very low prices, and the expected profit for the producer is therefore lower than for other products (e.g., drugs for treatment) competing for the same resources. However, while difficult to model when risks are unclear, it would be interesting to conduct more comprehensive and long-term cost-benefit analyses concerning the personal health impacts and the social and economic costs versus potential benefits of vaccine compared to treatment strategies for specific agents of interest.
- The added cost of the large clinical trials needed to address potential wide use including in diverse populations.
- The presence of advocacy groups with various points of view.
- A fair amount of concern about possible adverse effects of vaccines, ranging from specific disease issues to more general anti-vaccine sentiment on the part of a proportion of the public.
- A mistrust of government and industry
- Product liability issues.
Scientific Challenges
- Lack of historical or recent precedents for vaccines against many pathogens, which makes it difficult to establish good surrogates.
- The potential for genetic or other manipulation of antigenic determinants. (Although this is presently more difficult in many cases to engineer than antibacterial resistance.)
- The potential complications of live vaccine administration to increasing immunocompromised populations.
- The intense flow of resources demanded by urgent perceived needs (sometimes referred to as the “disease du jour” phenomenon), in contrast to the more normal lengthy product development cycle.
The FDA Response
There are several regulatory approaches and mechanisms that the FDA has employed in an attempt to safely speed up product availability and licensure:
- Early and frequent consultation between the sponsor producing the product, the potential end users (e.g., health officials and providers in the military and civilian sectors), and the FDA is very resource-intensive but important. This kind of up-front investment can greatly improve the product development process by identifying creative study designs, recognizing factors that are normally not anticipated in developing a product, and reducing misunderstandings and the likelihood of unwelcome surprises. Early dialogue also increases accountability.
- Emergency use under IND (investigational new drug status) allows rapid access to products that have not yet completed requirements for licensure. INDs require acceptable evidence of safety; a reasonable though not necessarily formally proven scientific basis for efficacy; a favorable nsk:benefit ratio; and an intent to license. While allowing availability of potentially lifesavmg products, a disadvantage to emergency use under this rule is that the product is not licensed, which not only reflects the true scientific limitations of the data but also raises important issues about public perception.
- Fast track processes can speed up the review process for products that will provide meaningful therapeutic benefits compared to existing therapies for serious or life-threatening illnesses. Fast track allows the FDA to review information as it becomes available and as the sponsor submits it.
- Accelerated approval through the use of surrogate end points to demonstrate benefit. The use of CD4 cells for assessment of antiviral treatment of HIV was one of the first surrogates to be approved under this rule. For bioterrorist agents, protective antibody levels for a vaccine or immunoglobulin could serve as potential surrogate end points. Clinical end points can also be utilized. There still must be good post-licensure studies to demonstrate the effects on disease outcomes and to collect additional safety information, and the FDA can place restrictions on use and promotion and even withdraw the product if agreements are violated or the product proves unsafe or ineffective. Thus far, this process has worked fairly well although, once a product is licensed, or if a disease is rare, it may be difficult to obtain patients for studies, and sponsors sometimes are unable or unmotivated to fulfill their commitments. But because most accelerated approval products also receive priority review, this process can allow for rapid approval of a product based on more limited and simpler-to-obtain clinical data than may be the case with large, randomized control trials and/or longer-term endpoints.
- Priority review is applied when a product is considered a significant advance or will be used for serious or life-threatening illness.
- Approval under the forthcoming “Animal Rule” has very important biodefense implications. In fact, the rule is specifically oriented to drugs or biologics that reduce or prevent serious or life threatening conditions caused by exposure to lethal or disabling toxic, chemical, biologic, or nuclear threats. The products should be expected to provide a meaningful therapeutic benefit over existing treatments. Human efficacy trials should either be not feasible or unethical, and the use of the animal efficacy data should be scientifically appropriate. In this proposed rule, the end point should be related to the desired benefit in humans, usually a significant outcome such as mortality or major morbidity. Clinical studies in representative populations are still needed, however, both for establishing pharmacokinetics (including, in the case of many vaccines, immunogenicity) and for assessing safety. Such studies are critical because civilian populations often include vulnerable or pharmacokinetically variable subsets. Finally, similar to the fast track and accelerated approvals, the animal rule has post-marketing and labeling commitments and restrictions. It does not apply if the product could be approved based on any other standard in FDA's regulation. It is a rule of last resort, but it certainly would be applicable to many of the situations that have been described in this workshop.
In addition to its regulatory responsibilities, the FDA's Center for Biologics conducts a significant amount of biodefense-related research, supporting approximately sixty ongoing projects that are directly relevant to identified high threat agents. The general goal is to meet otherwise unmet research needs, often with regulatory implications. Examples include how to better determine potency; defining immune and other correlates of protection; how to make safer and purer products (including characterization of the safety of cell substrates and detection of adventitious agents); better assessment of adverse events and efficacy under conditions of use, and studies which allow the agency to make regulations more scientific and less “defensive.” These types of research can benefit not only the public, but also multiple companies across industry, but are often not performed by a given sponsor as they may not provide a direct and/or immediate benefit. Furthermore, through its research and related scientific interactions, the center maintains the type of cutting edge expertise that is increasingly needed for dealing intelligently and proactively with evolving products and their underlying biotechnology. This expertise and confidence fosters the science-based objectivity necessary for anticipating and/or reacting appropriately to the issues raised during the development of a product which, ultimately, accelerates the regulatory and licensure process.
By maintaining its scientific, objective regulatory stance, the FDA can increase confidence in the likely efficacy of products primarily approved based on surrogate/animal data and reduce the likelihood of serious adverse events. The FDA brings several other unique attributes to the product development process as well, including:
- Knowledge of scientific and industrial capabilities, which is very helpful when it is necessary to identify people with specific expertise. This includes knowledge of emerging technologies which are cross-cutting among diverse products that nobody else may have the opportunity to see; knowledge of manufacturing capabilities; and knowledge of potential new uses of both licensed and investigational products, for example anti-sepsis and immune modulators.
- Day-to-day participation in what it takes to develop a product, including clinical trials, quality assurance, adverse event monitoring, timelines, etc.
- A unique ability to match product needs to industrial and academic capabilities. Much of this is informal, but it can be very helpful in getting the job done well.
However, there are several things that the FDA cannot do. FDA cannot
- provide monetary or tax incentives;
- assure that anyone will make a product;
- sponsor or directly assume the burden of product development, since this would be a conflict of interest;
- provide indemnification or compensation for injuries;
Furthermore, while the prelicensure process can provide reasonable assurances about the degree of safety and effectiveness, FDA cannot
- guarantee absolute safety;
- guarantee human efficacy under field conditions based on non-human data such as animal studies or surrogate endpoints (or, for that matter, based on efficacy observed in the controlled setting of a clinical trial).
In addition to expedited regulatory pathways, as well as orphan drug status, there are several potential incentives—both push and pull—which are outside the mission of FDA but that could be evaluated with respect to their potential to stimulate product development. Push incentives, which could be considered where markets are small or uncertain, could include:
- direct financial awards or contracts;
- tax credits;
- enhanced exclusivity;
- partnerships in product development; and
- research and development assistance to reduce the financial sting and risk of product development.
Possible pull incentives, which are probably more valuable, include:
- known markets;
- longer term financial contracts;
- defining prices that more accurately reflect known and potential public health benefit, which will require more economic discussion and modeling; and
- where possible, developing dual or multiple use products/concepts which can used not just for meeting bioterrorism needs but also for enhancing general public health and medical care.
In summary, FDA and CBER are highly committed to working with multiple partners in and outside of government to help in meeting the challenges posed by bioterrorism. Especially in times of threat and crisis, there is a need for a responsive, yet independent and science-based regulatory process. Relevant research and expertise remains critical in meeting the challenge. Existing laws and regulations can help facilitate product development in a timely manner. There are significant financial disincentives which have and may continue to impede the industrial development of some needed products where markets may be small or uncertain. Careful and open communication with the public about what is and is not known about proposed bioterrorism responses using new and existing products is critical not only in responding to specific threats and protecting the public but also in maintaining confidence in and support for the public health system as a whole.
- MOVING THE VACCINE AGENDA FORWARD: OBSTACLES AND OPPORTUNITIES
The vaccine industry is highly concentrated with only four major manufacturers providing more than three quarters of the market. Even within these four mam manufacturers production capacity may be insufficient for unseen circumstances or urgent need, as has been demonstrated by shortages of DT Acellular pertussis vaccine and pneumococcal conjugate vaccine. Following September 11th, all four major manufacturers expressed interest in biodefense/military vaccines. But how long will patriotism sustain this interest? The industry is both high risk (e.g., the rotavirus vaccine which had to be withdrawn from use) and risk adverse (e.g., certain vaccines for pregnant women have yet to be developed). There are several factors that impede vaccine product development:
- The vaccine industry is market-driven, and the major manufacturers are simply not interested if the market is insufficient.
- The industry is highly regulated, and perceived regulatory hurdles can impede product development.
- Intellectual property conflicts can prevent companies from developing products.
- Negative marketing assessment impedes vaccine production. Marketing departments often make unchecked predictions.
- An uncertain or dubious proof of concept creates reluctance to develop a particular new product.
- Biohazard to personnel, especially with regards to biodefense vaccines, can create reluctance.
The production of an adequate biodefense vaccine supply will depend on many factors:
- Development of biodefense vaccines must be in collaboration with DoD, NIH, CDC, and other agencies with national interests. With regard to the development of a new smallpox vaccine, for example, efficacy is an important issue that requires the use of monkey models for which the vaccine industry must turn to DoD or NIH.
- Development of biodefense vaccines requires better adverse reaction surveillance. With the military anthrax vaccine, for example, initially there were no data to support the claim that the vaccine was indeed safe. New vaccines must have built-in surveillance in order to discredit unsubstantiated claims about adverse reactions based on anecdotal data.
- New vaccine production requires that liability indemnification be guaranteed. One possible solution would be to place these vaccines on a list of compensable vaccines. We need to create a no-fault system which indemnifies companies against non-negligent harm but also provides some relief for injured individuals.
- Access to new technology would likely stimulate more interest in vaccine development. For example, a number of companies have pursued DNA vaccines because they are an interesting new technology which could likely be applied to a number of different targets. After all, the mam role of the vaccine industry is not to conduct basic research but apply basic research to the development of new products.
- Finally, vaccine production priorities need to be set and somebody authorized to say “this is the top priority.” For example, Gary Nabel has presented some very elegant work on an Ebola vaccine. But what does intelligence say is the real risk of Ebola? Should a virus that cannot be spread as an aerosol be considered a priority?
In summary, the major vaccine manufacturers will not be able to provide all of the needed biodefense vaccines. However, they should be asked to play a significant role. That role will be facilitated by clear directions, clear priority setting, and a tight collaboration between industry and government in order to move animal and clinical testing forward.
A. Johnson-Winegar
- Grabenstein JD, Wilson JP. Are Vaccines Safe? Risk Communication Applied to Vaccination. Hospital Pharmacy. 1999; 34 (6):713–729.
- Institute of Medicine. Interaction of Drugs, Biologics, and Chemicals in U.S. Military Forces. Washington, D.C.: National Academy Press; 1996. [ PubMed : 25121292 ]
- Use of Anthrax Vaccine in the United States—Recommendations of the Advisory Committee on Immunization Practices. Morbidity and Mortality Weekly Report. 2000 December 15; 49 (RR15):1–20. [ PubMed : 11145529 ]
- Balter M. Science. 2000; 290 :923–925. [ PubMed : 11184732 ]
- Colebunders R, Borchert M. Journal of Infections. 2000; 40 :16–20. [ PubMed : 10762106 ]
- Malashkevich VN, Schneider BJ, McNally ML, Milhollen MA, Pang JX, Kim PS. Proceeding of the National Academy of Sciences, U S A. 1999; 96 :2662–2667. [ PMC free article : PMC15825 ] [ PubMed : 10077567 ]
- Peters CJ, Sanchez A, Rollin PE, Ksiazek TG, Murphy FA. Fields Virology. Fields BN, Knipe DM, Howley PM, editors. Lippincott-Raven; Philadelphia: 1996. pp. 1161–1176.
- Peters CJ, Khan AS. Current Topics in Microbiology and Immunology. Klenk HD, editor. Vol. 235. Springer-Verlag; Berlin: 1999. pp. 85–95. [ PubMed : 9893380 ]
- Sanchez A, Khan AS, Zaki SR, Nabel GJ, Ksiazek TG, Peters CJ. Fields Virology. Fields BN, Knipe DM, Howley PM, editors. Lippincott-Raven; Philadelphia: 1996. pp. 1279–1304.
- Sanchez A, Trappier SG, Mahy BWJ, Peters CJ, Nichol ST. Proceedings of the National Academy of Sciences, USA. 1996; 93 :3602–3607. [ PMC free article : PMC39657 ] [ PubMed : 8622982 ]
- Sullivan NJ, Sanchez A, Rollin PE, Yang ZY, Nabel GJ. Nature. 2000; 408 :605–609. [ PubMed : 11117750 ]
- Volchkov VE, Becker S, Volchkova VA, Ternovoj VA, Kotov AN, Netesov SV, Klenk HD. Virology. 1995; 214 :421–430. [ PubMed : 8553543 ]
- Watanabe S, Takada A, Watanabe T, Ito H, Kida H, Kawaoka Y. Journal of Virology. 2000; 74 :10194–10201. [ PMC free article : PMC102058 ] [ PubMed : 11024148 ]
- Weissenhorn W, Calder LJ, Wharton SA, Skehel JJ, Wiley DC. Proceedings of the National Academy of Sciences, USA. 1998; 95 :6032–6036. [ PMC free article : PMC27580 ] [ PubMed : 9600912 ]
- Weissenhorn W, Carfi A, Lee KH, Skehel JJ, Wiley DC. Molecular Cell. 1998; 5 :605–616. [ PubMed : 9844633 ]
- Xu L, Sanchez A, Yang Z, Zaki SR, Nabel EG, Nichol ST, Nabel GJ. Nature Medicine. 1998; 4 :37–42. [ PubMed : 9427604 ]
- Yang Z, Delgado R, Xu L, Todd RF, Nabel EG, Sanchez A, Nabel GJ. Science. 1998; 279 :1034–1037. [ PubMed : 9461435 ]
- Yang ZY, Duckers HJ, Sullivan NJ, Sanchez A, Nabel EG, Nabel GJ. Nature Medicine. 2000; 6 :886–889. [ PubMed : 10932225 ]
This statement reflects the professional view of the author and should not be construed as an official position of the Department of Health and Human Services.
The information provided in this paper reflects the professional view of the author and not an official position of the U.S. Department of Defense.
This statement reflects the professional view of the author and should not be construed as an official position of the National Institute for Allergy and Infectious Diseases, National Institutes of Health.
This statement reflects the professional view of the author and should not be construed as an official position of the Food and Drug Administration.
- Cite this Page Institute of Medicine (US) Forum on Emerging Infections; Knobler SL, Mahmoud AAF, Pray LA, editors. Biological Threats and Terrorism: Assessing The Science and Response Capabilities: Workshop Summary. Washington (DC): National Academies Press (US); 2002. 3, Vaccines: Research, Development, Production, and Procurement Issues.
- PDF version of this title (5.5M)
In this Page
- VACCINES FOR THREATENING AGENTS: ENSURING THE AVAILABILITY OF COUNTERMEASURES FOR BIOTERRORISM
- THE DEPARTMENT OF DEFENSE AND THE DEVELOPMENT AND PROCUREMENT OF VACCINES AGAINST DANGEROUS PATHOGENS: A ROLE IN THE MILITARY AND CIVILIAN SECTOR?
- APPLICATIONS OF MODERN TECHNOLOGY TO EMERGING INFECTIONS AND DISEASE DEVELOPMENT: A CASE STUDY OF EBOLA VIRUS
- MEETING THE REGULATORY AND PRODUCT DEVELOPMENT CHALLENGES FOR VACCINES AND OTHER BIOLOGICS TO ADDRESS TERRORISM
Other titles in this collection
- The National Academies Collection: Reports funded by National Institutes of Health
Related information
- PMC PubMed Central citations
- PubMed Links to PubMed
Recent Activity
- Vaccines: Research, Development, Production, and Procurement Issues - Biological... Vaccines: Research, Development, Production, and Procurement Issues - Biological Threats and Terrorism
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers
Using pulp and paper waste to scrub carbon from emissions
Researchers at McGill University have come up with an innovative approach to improve the energy efficiency of carbon conversion, using waste material from pulp and paper production.
The technique they've pioneered using the Canadian Light Source at the University of Saskatchewan not only reduces the energy required to convert carbon into useful products, but also reduces overall waste in the environment.
"We are one of the first groups to combine biomass recycling or utilization with CO 2 capture," said Ali Seifitokaldani, Assistant Professor in the Department of Chemical Engineering and Canada Research Chair (Tier II) in Electrocatalysis for Renewable Energy Production and Conversion. The research team, from McGill's Electrocatalysis Lab, published their findings in the journal RSC Sustainability .
Capturing carbon emissions is one of the most exciting emerging tools to fight climate change. The biggest challenge is figuring out what to do with the carbon once the emissions have been removed, especially since capturing CO 2 can be expensive. The next hurdle is that transforming CO 2 into useful products takes energy. Researchers want to make the conversion process as efficient and profitable as possible.
- Energy and Resources
- Energy Technology
- Energy and the Environment
- Environmental Science
- Renewable Energy
- Global Warming
- Hazardous waste
- Photosynthesis
- Climate change mitigation
- Radioactive waste
- Carbon cycle
- Carbon dioxide
Story Source:
Materials provided by McGill University . Note: Content may be edited for style and length.
Journal Reference :
- Roger Lin, Haoyan Yang, Hanyu Zheng, Mahdi Salehi, Amirhossein Farzi, Poojan Patel, Xiao Wang, Jiaxun Guo, Kefang Liu, Zhengyuan Gao, Xiaojia Li, Ali Seifitokaldani. Efficient integration of carbon dioxide reduction and 5-hydroxymethylfurfural oxidation at high current density . RSC Sustainability , 2024; 2 (2): 445 DOI: 10.1039/D3SU00379E
Cite This Page :
Explore More
- Pregnancy Accelerates Biological Aging
- Tiny Plastic Particles Are Found Everywhere
- What's Quieter Than a Fish? A School of Them
- Do Odd Bones Belong to Gigantic Ichthyosaurs?
- Big-Eyed Marine Worm: Secret Language?
- Unprecedented Behavior from Nearby Magnetar
- Soft, Flexible 'Skeletons' for 'Muscular' Robots
- Toothed Whale Echolocation and Jaw Muscles
- Friendly Pat On the Back: Free Throws
- How the Moon Turned Itself Inside Out
Trending Topics
Strange & offbeat.

IMAGES
VIDEO
COMMENTS
Vaccination Behavior Research Topics. Unraveling demand and supply effects on the up-take of influenza vaccinations. Point out new approaches to the seasonal flu vaccine. Exploring the impact of vaccination. Investigating patient experience with, and the use of, an electronic monitoring system to assess vaccination responses.
Introduction "The impact of vaccination on the health of the world's peoples is hard to exaggerate. With the exception of safe water, no other modality has had such a major effect on mortality reduction and population growth" (Plotkin and Mortimer, 1988).The development of safe and efficacious vaccination against diseases that cause substantial morbidity and mortality has been one of the ...
Metrics. Vaccines have revolutionized modern medicine by preventing infectious diseases and safeguarding public health. This Collection showcases cutting-edge research on advancements in vaccine ...
Tetanus vaccines: WHO position paper, February 2017 — recommendations. Vaccine 36, 3573-3575 ... (SAGE) and a National Institute for Health Research (NIHR) Senior Investigator. The views ...
The protective effects of vaccination and prior infection against severe Covid-19 are reviewed, with proposed directions for future research, including mucosal immunity and intermittent vaccine boo...
A recent test-negative design study in Canada showed positive findings regarding vaccine effectiveness with the BNT162b2, mRNA-1273, and ChAdOx1 nCoV-19 vaccines against symptomatic disease with ...
Vaccination is a powerful method of disease prevention that is relevant to people of all ages and in all countries, as the Covid-19 pandemic illustrates. Vaccination can improve people's chances ...
Vaccines are a clinical product that is composed of live or dead material from an infectious agent - bacterium, virus, fungus or parasite - that elicit protective immunity against the pathogen ...
Since the outbreak of the COVID-19 pandemic, there has been a rapid expansion in vaccine research focusing on exploiting the novel discoveries on the pathophysiology, genomics, and molecular biology of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. ... Studies on these topics are rapidly being conducted and ...
In conclusion, a plethora of papers on COVID-19 vaccine have been published in the last three years, indicating that this theme is currently hottest topic in academic community. The USA, the UK, and China made many contributions to COVID-19 vaccine research globally.
Background Despite reliable evidence-based research supporting the COVID-19 vaccines, population-wide confidence and trust remain limited. We sought to expand prior knowledge about COVID-19 vaccine perceptions, while determining which population groups are at greatest risk for not getting a vaccine. Methods Study participants in the U.S. (79% female, median age group 46-60 years) were ...
To date, coronavirus disease 2019 (COVID-19) becomes increasingly fierce due to the emergence of variants. Rapid herd immunity through vaccination is needed to block the mutation and prevent the emergence of variants that can completely escape the immune surveillance. We aimed to systematically evaluate the effectiveness and safety of COVID-19 vaccines in the real world and to establish a ...
The COVID-19 pandemic has created a new reality where individuals are faced with a previously unknown disease and its effects, providing a unique opportunity to investigate vaccine attitudes during a period of heightened disease salience. The present research reports findings from a longitudinal study conducted during the COVID-19 health crisis ...
Promoting Public Health with Blunt Instruments: Evidence from Vaccine Mandates. Rahi Abouk, John S. Earle, Johanna Catherine Maclean & Sungbin Park. Working Paper 32286. DOI 10.3386/w32286. Issue Date March 2024. We study the effect of mandates requiring COVID-19 vaccination among healthcare industry workers adopted in 2021 in the United States.
A two-dose regimen of BNT162b2 (30 μg per dose, given 21 days apart) was found to be safe and 95% effective against Covid-19. The vaccine met both primary efficacy end points, with more than a 99 ...
There is no question that the current vaccines are effective and safe. The risk of severe reaction to a COVID-19 jab, say researchers, is outweighed by the protection it offers against the deadly ...
This 2023 White Paper encompasses recommendations emerging from the extensive discussions held during the workshop and is aimed to provide the bioanalytical community with key information and practical solutions on topics and issues addressed, in an effort to enable advances in scientific excellence, improved quality and better regulatory ...
This paper offers the first review study that fills this gap, paying particular attention to data and practical measurement challenges. It compares the World Bank's recently developed Statistical Performance Indicators and Index with other widely used indexes, such as the Open Data Inventory index, the Global Data Barometer index, and other ...
1. A phase 1/2 randomized, placebo-controlled, multicentre study to evaluate the safety and immunogenicity of COVID-19 vaccine in healthy adults. RBD SARS-CoV-2 HBsAg VLP vaccine, administered at two dose amounts 5 mcg and 25 mcg, by intramuscular injection by investigators during an in-clinic visit. RBD-HBsAg VLPs.
A research group in Japan has discovered that writing down one's reaction to a negative incident on a piece of paper and then shredding it or throwing it away reduces feelings of anger.
Vaccine platforms and vaccines for emerging infectious diseases. Vaccines are the cornerstone of the management of infectious disease outbreaks and are the surest means to defuse pandemic and ...
A Discrimination Report Card. Patrick M. Kline, Evan K. Rose & Christopher R. Walters. Working Paper 32313. DOI 10.3386/w32313. Issue Date April 2024. We develop an empirical Bayes ranking procedure that assigns ordinal grades to noisy measurements, balancing the information content of the assigned grades against the expected frequency of ...
S.B. HalsteadN Engl J Med 2024;390:464-465. In 2019, the four serotypes of the mosquito-borne dengue virus (DENV) caused an estimated 56 million cases of disease and 5000 to 40,000 deaths in a ...
Major problems at school. When we asked teachers about a range of problems that may affect students who attend their school, the following issues top the list: Poverty (53% say this is a major problem at their school) Chronic absenteeism - that is, students missing a substantial number of school days (49%) Anxiety and depression (48%) One-in ...
Here are six key facts about Americans and TikTok, drawn from Pew Research Center surveys. A third of U.S. adults - including a majority of adults under 30 - use TikTok. Around six-in-ten U.S. adults under 30 (62%) say they use TikTok, compared with 39% of those ages 30 to 49, 24% of those 50 to 64, and 10% of those 65 and older. In a 2023 ...
Introduction. The development of an effective vaccine to protect against HIV is a longstanding global health need complicated by challenges inherent to HIV biology and to the execution of vaccine efficacy testing in the context of evolving biomedical prevention interventions. This review describes lessons learnt from previous efficacy trials ...
In the past decade, VH has become a key topic of research in various fields, following rises in vaccine-preventable diseases, the introduction of new vaccines, the spread of misinformation and ...
In a paper published in The Lancet Diabetes and Endocrinology last week, the authors showed a clear link between high GI and diabetes. The authors say: "The association between the glycaemic ...
Vaccines not only afford the best protection against infectious disease but can serve as strong deterrence factors as well. From a bioterrorist perspective, vaccine-resistant agents are more difficult to engineer than drug-resistant agents. But the potential market has been too small and uncertain to encourage the vaccine industry to make large investments in research, development, and ...
Using pulp and paper waste to scrub carbon from emissions. ScienceDaily . Retrieved April 8, 2024 from www.sciencedaily.com / releases / 2024 / 04 / 240408130636.htm