An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Allergy Asthma Clin Immunol


Treatment strategies for asthma: reshaping the concept of asthma management
Alberto papi.
1 Section of Cardiorespiratory and Internal Medicine, Department of Morphology, Surgery and Experimental Medicine, University of Ferrara, Ferrara, Italy
7 Respiratory Unit, Emergency Department, University Hospital S. Anna, Via Aldo Moro 8, 44124 Ferrara, Italy
Francesco Blasi
2 Internal Medicine Department, Respiratory Unit and Adult Cystic Fibrosis Center, Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico, Milan, Italy
3 Department of Pathophysiology and Transplantation, Università degli Studi di Milano, Milan, Italy
Giorgio Walter Canonica
4 Personalized Medicine Asthma & Allergy Clinic, Humanitas University & Istituto Clinico Humanitas, Milan, Italy
Luca Morandi
Luca richeldi.
5 Università Cattolica del Sacro Cuore, Fondazione Policlinico A. Gemelli IRCCS, Rome, Italy
Andrea Rossi
6 Respiratory Section, Department of Medicine, University of Verona, Verona, Italy
Associated Data
Not applicable.
Asthma is a common chronic disease characterized by episodic or persistent respiratory symptoms and airflow limitation. Asthma treatment is based on a stepwise and control-based approach that involves an iterative cycle of assessment, adjustment of the treatment and review of the response aimed to minimize symptom burden and risk of exacerbations. Anti-inflammatory treatment is the mainstay of asthma management. In this review we will discuss the rationale and barriers to the treatment of asthma that may result in poor outcomes. The benefits of currently available treatments and the possible strategies to overcome the barriers that limit the achievement of asthma control in real-life conditions and how these led to the GINA 2019 guidelines for asthma treatment and prevention will also be discussed.
Asthma, a major global health problem affecting as many as 235 million people worldwide [ 1 ], is a common, non-communicable, and variable chronic disease that can result in episodic or persistent respiratory symptoms (e.g. shortness of breath, wheezing, chest tightness, cough) and airflow limitation, the latter being due to bronchoconstriction, airway wall thickening, and increased mucus.
The pathophysiology of the disease is complex and heterogeneous, involving various host-environment interactions occurring at various scales, from genes to organ [ 2 ].
Asthma is a chronic disease requiring ongoing and comprehensive treatment aimed to reduce the symptom burden (i.e. good symptom control while maintaining normal activity levels), and minimize the risk of adverse events such as exacerbations, fixed airflow limitation and treatment side effects [ 3 , 4 ].
Asthma treatment is based on a stepwise approach. The management of the patient is control-based; that is, it involves an iterative cycle of assessment (e.g. symptoms, risk factors, etc.), adjustment of treatment (i.e. pharmacological, non-pharmacological and treatment of modifiable risk factors) and review of the response (e.g. symptoms, side effects, exacerbations, etc.). Patients’ preferences should be taken into account and effective asthma management should be the result of a partnership between the health care provider and the person with asthma, particularly when considering that patients and clinicians might aim for different goals [ 4 ].
This review will discuss the rationale and barriers to the treatment of asthma, that may result in poor patient outcomes. The benefits of currently available treatments and the possible strategies to overcome the barriers that limit the achievement of asthma control in real-life situations will also be discussed.
The treatment of asthma: where are we? Evolution of a concept
Asthma control medications reduce airway inflammation and help to prevent asthma symptoms; among these, inhaled corticosteroids (ICS) are the mainstay in the treatment of asthma, whereas quick-relief (reliever) or rescue medicines quickly ease symptoms that may arise acutely. Among these, short-acting beta-agonists (SABAs) rapidly reduce airway bronchoconstriction (causing relaxation of airway smooth muscles).
National and international guidelines have recommended SABAs as first-line treatment for patients with mild asthma, since the Global Initiative for Asthma guidelines (GINA) were first published in 1995, adopting an approach aimed to control the symptoms rather than the underlying condition; a SABA has been the recommended rescue medication for rapid symptom relief. This approach stems from the dated idea that asthma symptoms are related to bronchial smooth muscle contraction (bronchoconstriction) rather than a condition concomitantly caused by airway inflammation. In 2019, the GINA guidelines review (GINA 2019) [ 4 ] introduced substantial changes overcoming some of the limitations and “weaknesses” of the previously proposed stepwise approach to adjusting asthma treatment for individual patients. The concept of an anti-inflammatory reliever has been adopted at all degrees of severity as a crucial component in the management of the disease, increasing the efficacy of the treatment while lowering SABA risks associated with patients’ tendency to rely or over-rely on the as-needed medication.
Until 2017, the GINA strategy proposed a pharmacological approach based on a controller treatment (an anti-inflammatory, the pillar of asthma treatment), with a SABA as an additional rescue intervention. The reliever, a short-acting bronc hodilator, was merely an addendum , a medication to be used in case the real treatment (the controller) failed to maintain disease control: SABAs effectively induce rapid symptom relief but are ineffective on the underlying inflammatory process. Based on the requirement to achieve control, the intensity of the controller treatment was related to the severity of the disease, varying from low-dose ICS to combination low-dose ICS/long-acting beta-agonist (LABA), medium-dose ICS/LABA, up to high-dose ICS/LABA, as preferred controller choice, with a SABA as the rescue medication. As a result, milder patients were left without any anti-inflammatory treatment and could only rely on SABA rescue treatment.
Poor adherence to therapy is a major limitation of a treatment strategy based on the early introduction of the regular use of controller therapy [ 5 ]. Indeed, a number of surveys have highlighted a common pattern in the use of inhaled medication [ 6 ], in which treatment is administered only when asthma symptoms occur; in the absence of symptoms, treatment is avoided as patients perceive it as unnecessary. When symptoms worsen, patients prefer to use reliever therapies, which may result in the overuse of SABAs [ 7 ]. Indirect evidence suggests that the overuse of beta-agonists alone is associated with increased risk of death from asthma [ 8 ].
In patients with mild persistent disease, low-dose ICS decreases the risk of severe exacerbations leading to hospitalization and improves asthma control [ 9 ]. When low-dose ICS are ineffective in controlling the disease (Step 3 of the stepwise approach), a combination of low-dose ICS with LABA maintenance was the recommended first-choice treatment, plus as-needed SABA [ 3 , 10 ]. Alternatively, the combination low-dose ICS/LABA (formoterol) was to be used as single maintenance and reliever treatment (SMART). The SMART strategy containing the rapid-acting formoterol was recommended throughout GINA Steps 3 to 5 based on solid clinical-data evidence [ 3 ].
The addition of a LABA to ICS treatment reduces both severe and mild asthma exacerbation rates, as shown in the one-year, randomized, double-blind, parallel-group FACET study [ 11 ]. This study focused on patients with persistent asthma symptoms despite receiving ICS and investigated the efficacy of the addition of formoterol to two dose levels of budesonide (100 and 400 µg bid ) in decreasing the incidence of both severe and mild asthma exacerbations. Adding formoterol decreased the incidence of both severe and mild asthma exacerbations, independent of ICS dose. Severe and mild exacerbation rates were reduced by 26% and 40%, respectively, with the addition of formoterol to the lower dose of budesonide; the corresponding reductions were 63% and 62%, respectively, when formoterol was added to budesonide at the higher dose.
The efficacy of the ICS/LABA combination was confirmed in the post hoc analysis of the FACET study, in which patients were exposed to a combination of formoterol and low-dose budesonide [ 12 ]. However, such high levels of asthma control are not achieved in real life [ 5 ]. An explanation for this is that asthma is a variable condition and this variability might include the exposure of patients to factors which may cause a transient steroid insensitivity in the inflammatory process. This, in turn, may lead to an uncontrolled inflammatory response and to exacerbations, despite optimal controller treatment. A typical example of this mechanism is given by viral infections, the most frequent triggers of asthma exacerbations. Rhinoviruses, the most common viruses found in patients with asthma exacerbations, interfere with the mechanism of action of corticosteroids making the anti-inflammatory treatment transiently ineffective. A transient increase in the anti-inflammatory dose would overcome the trigger-induced anti-inflammatory resistance, avoiding uncontrolled inflammation leading to an exacerbation episode [ 13 – 15 ].
Indeed, symptoms are associated with worsening inflammation and not only with bronchoconstriction. Romagnoli et al. showed that inflammation, as evidenced by sputum eosinophilia and eosinophilic markers, is associated with symptomatic asthma [ 16 ]. A transient escalation of the ICS dose would prevent loss of control over inflammation and decrease the risk of progression toward an acute episode. In real life, when experiencing a deterioration of asthma control, patients self-treat by substantially increasing their SABA medication (Fig. 1 ); it is only subsequently that they (modestly) increase the maintenance treatment [ 17 ].

Mean use of SABA at different stages of asthma worsening. Patients have been grouped according to maintenance therapy shown in the legend. From [ 17 ], modified
As bronchodilators, SABAs do not control the underlying inflammation associated with increased symptoms. The “as required” use of SABAs is not the most effective therapeutic option in controlling a worsening of inflammation, as signaled by the occurrence of symptoms; instead, an anti-inflammatory therapy included in the rescue medication along with a rapid-acting bronchodilator could provide both rapid symptom relief and control over the underlying inflammation. Thus, there is a need for a paradigm shift, a new therapeutic approach based on the rescue use of an inhaled rapid-acting beta-agonist combined with an ICS: an anti-inflammatory reliever strategy [ 18 ].
The symptoms of an exacerbation episode, as reported by Tattersfield and colleagues in their extension of the FACET study, increase gradually before the peak of the exacerbation (Fig. 2 ); and the best marker of worsening asthma is the increased use of rescue beta-agonist treatment that follows exactly the pattern of worsening symptomatology [ 19 ]. When an ICS is administered with the rescue bronchodilator, the patient would receive anti-inflammatory therapy when it is required; that is, when the inflammation is uncontrolled, thus increasing the efficiency of the anti-inflammatory treatment.

Percent variation in symptoms, rescue beta-agonist use and peak expiratory flow (PEF) during an exacerbation. In order to allow comparison over time, data have been standardized (Day-14 = 0%; maximum change = 100%)
(From [ 19 ])
Barriers and paradoxes of asthma management
A number of barriers and controversies in the pharmacological treatment of asthma have prevented the achievement of effective disease management [ 20 ]. O’Byrne and colleagues described several such controversies in a commentary published in 2017, including: (1) the recommendation in Step 1 of earlier guidelines for SABA bronchodilator use alone, despite asthma being a chronic inflammatory condition; and (2) the autonomy given to patients over perception of need and disease control at Step 1, as opposed to the recommendation of a fixed-dose approach with treatment-step increase, regardless of the level of symptoms [ 20 ]. Other controversies outlined were: (3) a difficulty for patients in understanding the recommendation to minimize SABA use at Step 2 and switch to a fixed-dose ICS regimen, when they perceive SABA use as more effective; (4) apparent conflicting safety messages within the guidelines that patient-administered SABA monotherapy is safe, but patient-administered LABA monotherapy is not; and (5) a discrepancy as to patients’ understanding of “controlled asthma” and their symptom frequency, impact and severity [ 20 ].
Controversies (1) and (2) can both establish an early over-dependence on SABAs. Indeed, asthma patients freely use (and possibly overuse) SABAs as rescue medication. UK registry data have recently suggested SABA overuse or overreliance may be linked to asthma-related deaths: among 165 patients on short-acting relievers at the time of death, 56%, 39%, and 4% had been prescribed > 6, > 12, and > 50 SABA inhalers respectively in the previous year [ 21 ]. Registry studies have shown the number of SABA canisters used per year to be directly related to the risk of death in patients with asthma. Conversely, the number of ICS canisters used per year is inversely related to the rate of death from asthma, when compared with non-users of ICS [ 8 , 22 ]. Furthermore, low-dose ICS used regularly are associated with a decreased risk of asthma death, with discontinuation of these agents possibly detrimental [ 22 ].
Other barriers to asthma pharmacotherapy have included the suggestion that prolonged treatment with LABAs may mask airway inflammation or promote tolerance to their effects. Investigating this, Pauwels and colleagues found that in patients with asthma symptoms that were persistent despite taking inhaled glucocorticoids, the addition of regular treatment with formoterol to budesonide for a 12-month period did not decrease asthma control, and improved asthma symptoms and lung function [ 11 ].
Treatment strategies across all levels of asthma severity
Focusing on risk reduction, the 2014 update of the GINA guidelines recommended as-needed SABA for Step 1 of the stepwise treatment approach, with low-dose ICS maintenance therapy as an alternative approach for long-term anti-inflammatory treatment [ 23 ]. Such a strategy was only supported by the evidence from a post hoc efficacy analysis of the START study in patients with recently diagnosed mild asthma [ 24 ]. The authors showed that low-dose budesonide reduced the decline of lung-function over 3 years and consistently reduced severe exacerbations, regardless of symptom frequency at baseline, even in subjects with symptoms below the then-threshold of eligibility for ICS [ 24 ]. However, as for all post hoc analyses, the study by Reddel and colleagues does not provide conclusive evidence and, even so, their results could have questionable clinical significance for the management of patients with early mild asthma. To be effective, this approach would require patients to be compliant to regular twice-daily ICS for 10 years to have the number of exacerbations reduce by one. In real life, it is highly unlikely that patients with mild asthma would adhere to such a regular regimen [ 25 ].
The 2016 update to the GINA guidelines lowered the threshold for the use of low-dose ICS (GINA Step 2) to two episodes of asthma symptoms per month (in the absence of any supportive evidence for the previous cut-off). The objective was to effectively increase the asthma population eligible to receive regular ICS treatment and reduce the population treated with a SABA only, given the lack of robust evidence of the latter’s efficacy and safety and the fact that asthma is a variable condition characterized by acute exacerbations [ 26 ]. Similarly, UK authorities recommended low-dose ICS treatment in mild asthma, even for patients with suspected asthma, rather than treatment with a SABA alone [ 10 ]. However, these patients are unlikely to have good adherence to the regular use of an ICS. It is well known that poor adherence to treatment is a major problem in asthma management, even for patients with severe asthma. In their prospective study of 2004, Krishnan and colleagues evaluated the adherence to ICS and oral corticosteroids (OCS) in a cohort of patients hospitalized for asthma exacerbations [ 27 ]. The trend in the data showed that adherence to ICS and OCS treatment in patients dropped rapidly to reach nearly 50% within 7 days of hospital discharge, with the rate of OCS discontinuation per day nearly double the rate of ICS discontinuation per day (− 5.2% vs. − 2.7%; p < 0.0001 respectively, Fig. 3 ), thus showing that even after a severe event, patients’ adherence to treatment is suboptimal [ 27 ].

Use of inhaled (ICS) and oral (OCS) corticosteroids in patients after hospital discharge among high-risk adult patients with asthma. The corticosteroid use was monitored electronically. Error bars represent the standard errors of the measured ICS and OCS use
(From [ 27 ])
Guidelines set criteria with the aim of achieving optimal control of asthma; however, the attitude of patients towards asthma management is suboptimal. Partridge and colleagues were the first in 2006 to evaluate the level of asthma control and the attitude of patients towards asthma management. Patients self-managed their condition using their medication as and when they felt the need, and adjusted their treatment by increasing their intake of SABA, aiming for an immediate relief from symptoms [ 17 ]. The authors concluded that the adoption of a patient-centered approach in asthma management could be advantageous to improve asthma control.
The concomitant administration of an as-needed bronchodilator and ICS would provide rapid relief while administering anti-inflammatory therapy. This concept is not new: in the maintenance and reliever approach, patients are treated with ICS/formoterol (fast-acting, long-acting bronchodilator) combinations for both maintenance and reliever therapy. An effective example of this therapeutic approach is provided in the SMILE study in which symptomatic patients with moderate to severe asthma and treated with budesonide/formoterol as maintenance therapy were exposed to three different as-needed options: SABA (terbutaline), rapid-onset LABA (formoterol) and a combination of LABA and ICS (budesonide/formoterol) [ 28 ]. When compared with formoterol, budesonide/formoterol as reliever therapy significantly reduced the risk of severe exacerbations, indicating the efficacy of ICS as rescue medication and the importance of the as-needed use of the anti-inflammatory reliever.
The combination of an ICS and a LABA (budesonide/formoterol) in one inhaler for both maintenance and reliever therapy is even more effective than higher doses of maintenance ICS and LABA, as evidenced by Kuna and colleagues and Bousquet and colleagues (Fig. 4 ) [ 29 , 30 ].

Comparison between the improvements in daily asthma control resulting from the use of budesonide/formoterol maintenance and reliever therapy vs. higher dose of ICS/LABA + SABAZ and steroid load for the two regimens
(Data from [ 29 , 30 ])
The effects of single maintenance and reliever therapy versus ICS with or without LABA (controller therapy) and SABA (reliever therapy) have been recently addressed in the meta-analysis by Sobieraj and colleagues, who analysed 16 randomized clinical trials involving patients with persistent asthma [ 31 ]. The systematic review supported the use of single maintenance and reliever therapy, which reduces the risk of exacerbations requiring systemic corticosteroids and/or hospitalization when compared with various strategies using SABA as rescue medication [ 31 ].
This concept was applied to mild asthma by the BEST study group, who were the first to challenge the regular use of ICS. A pilot study by Papi and colleagues evaluated the efficacy of the symptom-driven use of beclomethasone dipropionate plus albuterol in a single inhaler versus maintenance with inhaled beclomethasone and as-needed albuterol. In this six-month, double-blind, double-dummy, randomized, parallel-group trial, 455 patients with mild asthma were randomized to one of four treatment groups: an as-needed combination therapy of placebo bid plus 250 μg of beclomethasone and 100 μg of albuterol in a single inhaler; an as-needed albuterol combination therapy consisting of placebo bid plus 100 μg of albuterol; regular beclomethasone therapy, comprising beclomethasone 250 μg bid and 100 μg albuterol as needed); and regular combination therapy with beclomethasone 250 μg and albuterol 100 μg in a single inhaler bid plus albuterol 100 μg as needed.
The rescue use of beclomethasone/albuterol in a single inhaler was as efficacious as the regular use of inhaled beclomethasone (250 μg bid ) and it was associated with a lower 6-month cumulative dose of the ICS [ 32 ].
The time to first exacerbation differed significantly among groups ( p = 0.003), with the shortest in the as-needed albuterol and placebo group (Fig. 5 ). Figure 5 also shows equivalence between the as-needed combination therapy and the regular beclomethasone therapy. However, these results were not conclusive since the study was not powered to evaluate the effect of the treatment on exacerbations. In conclusion, as suggested by the study findings, mild asthma patients may require the use of an as-needed ICS and an inhaled bronchodilator rather than a regular treatment with ICS [ 32 ].

Kaplan Meier analysis of the time to first exacerbation (modified intention-to-treat population). First asthma exacerbations are shown as thick marks. As-needed albuterol therapy = placebo bid plus 100 μg of albuterol as needed; regular combination therapy = 250 μg of beclomethasone and 100 μg of albuterol in a single inhaler bid plus 100 μg of albuterol as needed; regular beclomethasone therapy = 250 μg of beclomethasone bid and 100 μg of albuterol as needed; as-needed combination therapy = placebo bid plus 250 μg of beclomethasone and 100 μg of albuterol in a single inhaler as needed
(From [ 32 ])
Moving forward: a new approach to the management of asthma patients
Nearly a decade after the publication of the BEST study in 2007, the use of this alternative therapeutic strategy was addressed in the SYGMA 1 and SYGMA 2 trials. These double-blind, randomized, parallel-group, 52-week phase III trials evaluated the efficacy of as-needed use of combination formoterol (LABA) and the ICS budesonide as an anti-inflammatory reliever in patients requiring GINA Step 2 treatment, with the current reliever therapy (e.g. as-needed SABA) or with low-dose maintenance ICS (inhaled budesonide bid ) plus as-needed SABA, administered as regular controller therapy [ 33 , 34 ].
The SYGMA 1 trial, which enrolled 3849 patients, aimed to demonstrate the superiority of the as-needed use of the combination budesonide/formoterol over as-needed terbutaline, as measured by the electronically-recorded proportion of weeks with well-controlled asthma [ 34 ]. The more pragmatic SYGMA 2 trial enrolled 4215 patients with the aim to demonstrate that the budesonide/formoterol combination is non-inferior to budesonide plus as-needed terbutaline in reducing the relative rate of annual severe asthma exacerbations [ 33 ]. Both trials met their primary efficacy outcomes. In particular, as-needed budesonide/formoterol was superior to as-needed SABA in controlling asthma symptoms (34.4% versus 31.1%) and preventing exacerbations, achieving a 64% reduction in exacerbations. In both trials, budesonide/formoterol as-needed was similar to budesonide maintenance bid at preventing severe exacerbations, with a substantial reduction of the inhaled steroid load over the study period (83% in the SYGMA 1 trial and 75% in the SYGMA 2 trial). The time to first exacerbation did not differ significantly between the two regimens; however, budesonide/formoterol was superior to SABA in prolonging the time to first severe exacerbation [ 33 , 34 ].
The double-blind, placebo-controlled design of the SYGMA trials does not fully address the advantages of anti-inflammatory reliever strategy in patients who often rely on SABAs for symptom relief, so to what extent the study findings could apply to real-life practice settings was unclear.
These limitations were overcome by the results of the Novel START study, an open-label, randomized, parallel-group, controlled trial designed to reflect real-world practice, which demonstrated the effectiveness in mild asthma of budesonide/formoterol as an anti-inflammatory reliever therapy [ 35 ].
In real-world practice, mild asthma patients are treated with an as-needed SABA reliever or with daily low-dose ICS maintenance therapy plus a SABA reliever. In the Novel START study, 668 patients with mild asthma were randomized to receive either as-needed albuterol 100 µg, two inhalations (SABA reliever as a continuation of the Step 1 treatment according to the 2017 GINA guidelines), budesonide 200 µg (ICS maintenance treatment) plus as-needed albuterol (Step 2 therapy of the GINA 2017 guidelines), or 200 µg/6 µg budesonide/formoterol as anti-inflammatory reliever therapy taken as-needed for a 52-week study period.
In this study, the rate of asthma exacerbations for budesonide/formoterol was lower compared with albuterol (51%) and similar to the twice-daily maintenance budesonide plus albuterol, despite a 52% reduction in the mean steroid dose with the single combination inhaler treatment [ 35 ]. In addition, severe exacerbation rate was lower with budesonide/formoterol as compared with as-needed albuterol and regular twice-daily budesonide. These data support the findings of the SYGMA 1 and 2 trials, highlighting the need for a critical re-examination of current clinical practice. Along with the results of the SYGMA trials, they provide convincing evidence of the advantages of the anti-inflammatory reliever strategy, particularly in real-life settings.
The SYGMA 1, SYGMA 2 and the novel START studies complete the picture of the treatment strategies for asthma at any degree of severity, including mild asthma. A growing body of evidence shows that an anti-inflammatory reliever strategy, when compared with all other strategies with SABA reliever, consistently reduces the rate of exacerbations across all levels of asthma severity (Fig. 6 ) [ 28 , 29 , 34 , 36 – 39 ].

Risk reduction of severe asthma attack of anti-inflammatory reliever versus SABA across all levels of asthma severity. Bud = budesonide; form = formoterol; TBH = turbohaler. Data from: 1: [ 36 ]; 2: [ 37 ]; 3: [ 38 ]; 4: [ 28 ]; 5: [ 29 ]; 6: [ 30 ]; 7: [ 34 ]
(Data source: [ 39 ])
This evidence set the ground (Fig. 7 ) for the release of the 2019 GINA strategy updates. The document provides a consistent approach towards the management of the disease and aims to avoid the overreliance and overuse of SABAs, even in the early course of the disease. The 2019 GINA has introduced key changes in the treatment of mild asthma: for safety reasons, asthmatic adults and adolescents should receive ICS-containing controller treatment instead of the SABA-only treatment, which is no longer recommended.

Timeline of key randomized controlled trials and meta-analyses providing the supporting evidence base leading to the Global Initiative for Asthma (GINA) 2019 guidelines. GINA global initiative for asthma, MART maintenance and reliever therapy, SMART single inhaler maintenance and reliever therapy
In Step 1 of the stepwise approach to adjusting asthma treatment, the preferred controller option for patients with fewer than two symptoms/month and no exacerbation risk factors is low-dose ICS/formoterol as needed. This strategy is indirectly supported by the results of the SYGMA 1 study which evaluated the efficacy and safety of budesonide/formoterol as needed, compared with as-needed terbutaline and budesonide bid plus as-needed terbutaline (see above). In patients with mild asthma, the use of an ICS/LABA (budesonide/formoterol) combination as needed provided superior symptom control to as-needed SABA, resulting in a 64% lower rate of exacerbations (p = 0.07) with a lower steroid dose (17% of the budesonide maintenance dose) [ 34 ]. The changes extend to the other controller options as well. In the 2017 GINA guidelines, the preferred treatment was as-needed SABA with the option to consider adding a regular low-dose ICS to the reliever. In order to overcome the poor adherence with the ICS regimen, and with the aim to reduce the risk of severe exacerbations, the 2019 GINA document recommends taking low-dose ICS whenever SABA is taken, with the daily ICS option no longer listed.
Previous studies including the TREXA study in children and adolescents [ 40 ], the BASALT study [ 41 ] and research conducted by the BEST study group [ 32 ] have already added to the evidence that a low-dose ICS with a bronchodilator is an effective strategy for symptom control in patients with mild asthma. A recently published study in African-American children with mild asthma found that the use of as-needed ICS with SABA provides similar asthma control, exacerbation rates and lung function measures at 1 year, compared with daily ICS controller therapy [ 42 ], adding support to TREXA findings that in children with well controlled, mild asthma, ICS used as rescue medication with SABA may be an efficacious step-down strategy [ 40 ].
In Step 2 of the stepwise approach, there are now two preferred controller options: (a) a daily low-dose ICS plus an as-needed SABA; and (b) as-needed low-dose ICS/formoterol. Recommendation (a) is supported by a large body of evidence from randomized controlled trials and observations showing a substantial reduction of exacerbation, hospitalization, and death with regular low-dose ICS [ 7 – 9 , 24 , 43 ], whereas recommendation (b) stems from evidence on the reduction or non-inferiority for severe exacerbations when as-needed low-dose ICS/formoterol is compared with regular ICS [ 33 , 34 ].
The new GINA document also suggests low-dose ICS is taken whenever SABA is taken, either as separate inhalers or in combination. This recommendation is supported by studies showing reduced exacerbation rates compared with taking a SABA only [ 32 , 40 ], or similar rates compared with regular ICS [ 32 , 40 , 41 ]. Low-dose theophylline, suggested as an alternative controller in the 2017 GINA guidelines, is no longer recommended.
Airway inflammation is present in the majority of patients with asthma, and although patients with mild asthma may have only infrequent symptoms, they face ongoing chronic inflammation of the lower airways and risk acute exacerbations. The GINA 2019 strategy recognizes the importance of reducing the risk of asthma exacerbations, even in patients with mild asthma (Steps 1 and 2) [ 4 ]. In this regard, the new recommendations note that SABA alone for symptomatic treatment is non-protective against severe exacerbation and may actually increase exacerbation risk if used regularly or frequently [ 4 ].
The reluctance by patients to regularly use an ICS controller means they may instead try and manage their asthma symptoms by increasing their SABA reliever use. This can result in SABA overuse and increased prescribing, and increased risk of exacerbations.
As part of the global SABINA (SABA use IN Asthma) observational study programme, a UK study examined primary care records to describe the pattern of SABA and ICS use over a 10-year period in 373,256 patients with mild asthma [ 44 ]. Results showed that year-to-year SABA prescribing was more variable than that of ICS indicating that, in response to fluctuations in asthma symptom control, SABA use was increased in preference to ICS use. Furthermore, more than 33% of patients were prescribed SABA inhalers at a level equivalent to around ≥ 3 puffs per week which, according to GINA, suggests inadequate asthma control.
The problem of SABA overuse is further highlighted by two studies [ 45 , 46 ], also as part of the SABINA programme. These analysed data from 365,324 patients in a Swedish cohort prescribed two medications for obstructive lung disease in any 12-month period (HERA).
The first study identified SABA overuse (defined as ≥ 3 SABA canisters a year) in 30% of patients, irrespective of their ICS use; 21% of patients were collecting 3–5 canisters annually, 7% were collecting 6–10, and 2% more than 11 [ 45 ]. Those patients who were overusing SABA had significantly more asthma exacerbations relative to those using < 3 canisters (20.0 versus 12.5 per 100 patient years; relative risk 1.60, 95% CI 1.57–1.63, p < 0.001). Moreover, patients overusing SABA and whose asthma was more severe (GINA Steps 3 and 4) had greater exacerbation risk compared with overusing patients whose asthma was milder (GINA Steps 1 and 2).
The second study found those patients using three or more SABA reliever canisters a year had an increased all-cause mortality risk relative to patients using fewer SABA canisters: hazard ratios after adjustment were 1.26 (95% CI 1.14–1.39) for 3–5 canisters annually, 1.67 (1.49–1.87) for 6–10 canisters, and 2.35 (2.02–2.72) for > 11 canisters, relative to patients collecting < 3 canisters annually [ 46 ].
The recently published PRACTICAL study lends further support to as-needed low-dose ICS/formoterol as an alternative option to daily low-dose ICS plus as-needed SABA, outlined in Step 2 of the guidelines [ 47 ]. In their one-year, open-label, multicentre, randomized, superiority trial in 890 patients with mild to moderate asthma, Hardy and colleagues found that the rate of severe exacerbations per patient per year (the primary outcome) was lower in patients who received as-needed budesonide/formoterol than in patients who received controller budesonide plus as-needed terbutaline (relative rate 0.69, 95% CI 0.48–1.00; p < 0.05). Indeed, they suggest that of these two treatment options, as-needed low-dose ICS/formoterol may be preferred over controller low-dose ICS plus as-needed SABA for the prevention of severe exacerbations in this patient population.
Step 3 recommendations have been left unchanged from 2017, whereas Step 4 treatment has changed from recommending medium/high-dose ICS/LABA [ 3 ] to medium-dose ICS/LABA; the high-dose recommendation has been escalated to Step 5. Patients who have asthma that remains uncontrolled after Step 4 treatment should be referred for phenotypic assessment with or without add-on therapy.
To summarise, the use of ICS medications is of paramount importance for optimal asthma control. The onset and increase of symptoms are indicative of a worsening inflammation leading to severe exacerbations, the risk of which is reduced by a maintenance plus as-needed ICS/LABA combination therapy. The inhaled ICS/bronchodilator combination is as effective as the regular use of inhaled steroids.
The efficacy of anti-inflammatory reliever therapy (budesonide/formoterol) versus current standard-of-care therapies in mild asthma (e.g. reliever therapy with a SABA as needed and regular maintenance controller therapy plus a SABA as-needed) has been evaluated in two randomized, phase III trials which confirmed that, with respect to as-needed SABA, the anti-inflammatory reliever as needed is superior in controlling asthma and reduces exacerbation rates, exposing the patients to a substantially lower glucocorticoid dose.
Conclusions
A growing body of evidence shows that anti-inflammatory reliever strategy is more effective than other strategies with SABA reliever in controlling asthma and reducing exacerbations across all levels of asthma severity. A budesonide/formoterol therapy exposes asthma patients to a substantially lower glucocorticoid dose while cutting the need for adherence to scheduled therapy.
Acknowledgements
The Authors thank Maurizio Tarzia and Gayle Robins, independent medical writers who provided editorial assistance on behalf of Springer Healthcare Communications. The editorial assistance was funded by AstraZeneca.
Abbreviations
Authors’ contributions.
AP, FB, GWC, LM, LR and AR contributed to writing. All authors read and approved the final manuscript.
No funding was received for this study. The editorial assistance was funded by AstraZeneca.
Availability of data and materials
Ethics approval and consent to participate, consent for publication, competing interests.
AP reports grants, personal fees, non-financial support and payment for advisory board membership, consultancy, payment for lectures, grants for research, and travel expenses reimbursement from Chiesi, AstraZeneca, GlaxoSmithKline, Boehringer Ingelheim, Mundipharma and Teva, and personal fees and non-financial support from Menarini, Novartis, Zambon and Sanofi.
FB reports having received in the last three years research grants as well as lecture or advisory board fees from: Alk-Abelló, AstraZeneca, Boehringer Ingelheim, Chiesi, Guidotti, Glaxo Smith Kline, Grifols, Menarini, Novartis, Sanofi, Valeas, Zambon.
GWC reports having received in the last 3 years research grants as well as lecture or advisory board fees from: A. Menarini, Alk-Abelló, AstraZeneca-Medimmune, Boehringer Ingelheim, Chiesi Farmaceutici, Genentech, Guidotti-Malesci, Glaxo Smith Kline, Hal Allergy, Merck Sharp & Dohme, Mundipharma, Novartis, Orion, Sanofi-Aventis, Sanofi Genzyme/Regeneron, Stallergenes-Greers, UCB Pharma, Uriach Pharma, Valeas.
LR Receipt of grants/research supports: Roche, Boehringer Ingelheim.
Receipt of honoraria or consultation fees: Boehringer Ingelheim, Roche, Biogen, FibroGen,
Sanofi-Aventis, Anthera, Promedior, ImmuneWorks, Asahi-Kasei, Bayer, Celgene, RespiVant,
Nitto, Bristol Myers Squibb, Prometic, Pliant Therapeutics, Toray, Global Blood Therapeutics,
Zambon, Veracyte, Acceleron, CSL Behring.
LM and AR reports no conflicts of interest in the last 3 years.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appointments at Mayo Clinic
Asthma treatment: 3 steps to better asthma control.
Follow this three-step approach to keep asthma symptoms under control and prevent asthma attacks.
The goals of asthma treatment are to limit symptoms, prevent asthma attacks and avoid side effects of asthma medicines. The following three steps can help you take control of your asthma treatment.
1. Follow your asthma action plan
Your health care team may work with you to create a written asthma action plan. This plan tells you how to make decisions every day and when to take your medicines. Following this plan is key to controlling your asthma.
A plan has three parts with color codes:
- Green. The green zone of the plan is for times you are feeling well and have no asthma symptoms. The plan tells you what dose of long-term control medicine to take every day. It also tells you how many puffs of a quick-relief inhaler to take before you exercise.
- Yellow. The yellow zone tells you what to do if you have symptoms. It explains when to use a quick-relief inhaler and how many puffs to take. It also describes what to do if your symptoms don't improve and when to call your care team.
- Red. The red zone tells you when to get emergency care if symptoms don't improve or if they worsen.
2. Use at-home lung tests
Your health care team may ask you to use a device that measures how well your lungs are working. This is called a lung function test.
Your asthma action plan includes instructions for when or how often you should do a lung function test. The plan also tells you what to do if the test shows your lungs aren't working well.
You may use one of these devices:
- Peak flow meter. This device measures how quickly you can force air out of your lungs. Peak flow readings are usually a percentage of how your lungs work at their best. This is called your personal best peak flow.
- Spirometer. A spirometer measures how much air your lungs can hold and how quickly you can breathe out. This measurement is called forced expiratory volume (FEV-1). Your FEV-1 measurement is compared with the typical FEV-1 for people who don't have asthma. As with your peak flow reading, this comparison is often given as a percentage. Your health care team will likely use this test during your office visits, but you may need to use a hand-held spirometer at home.
3. Keep an asthma diary
Keep an asthma diary every day. This information helps you keep track of your symptoms and helps you share accurate information with your health care team. Record the following information:
- Dose of long-term and quick-relief medicines you use each day.
- Description of symptoms.
- Severity and duration of symptoms.
- Time of day when symptoms occur.
- Possible triggers of symptoms, such as exercise or allergies.
- Difficulty with work, school, exercise or other day-to-day activities because of asthma symptoms.
- Results of a lung function test.
- Unscheduled appointments or urgent care for asthma.
Symptoms to record in your asthma diary include:
- Shortness of breath or coughing.
- Whistling sounds when you exhale, called wheezing.
- Disturbed sleep caused by shortness of breath, coughing or wheezing.
- Chest tightness or pain.
- Hay fever symptoms such as sneezing and runny nose.
Work with your health care team
You will likely meet with your care team regularly for checkups to see how you are doing. Bring your written asthma action plan and your asthma diary to every appointment. The information in your diary helps your asthma specialist know if your asthma is well controlled, poorly controlled or very poorly controlled.
Levels of asthma control in people 12 and older
If your asthma is well controlled, your provider may lower the dose of your medicines. If your asthma is poorly controlled or very poorly controlled, you may need to take different medicines or higher doses of medicine. These changes are recorded in your new asthma action plan.
You also may need to take steps to control triggers, such as increasing or changing allergy treatments. You may need to take steps to remove or avoid asthma triggers.
There is a problem with information submitted for this request. Review/update the information highlighted below and resubmit the form.
From Mayo Clinic to your inbox
Sign up for free and stay up to date on research advancements, health tips, current health topics, and expertise on managing health. Click here for an email preview.
Error Email field is required
Error Include a valid email address
To provide you with the most relevant and helpful information, and understand which information is beneficial, we may combine your email and website usage information with other information we have about you. If you are a Mayo Clinic patient, this could include protected health information. If we combine this information with your protected health information, we will treat all of that information as protected health information and will only use or disclose that information as set forth in our notice of privacy practices. You may opt-out of email communications at any time by clicking on the unsubscribe link in the e-mail.
Thank you for subscribing!
You'll soon start receiving the latest Mayo Clinic health information you requested in your inbox.
Sorry something went wrong with your subscription
Please, try again in a couple of minutes
- Fanta CH. An overview of asthma management. https://www.uptodate.com/contents/search. Accessed May 8, 2023.
- Asthma care quick reference: Diagnosing and managing asthma. National Heart, Lung, and Blood Institute. https://www.nhlbi.nih.gov/sites/default/files/media/docs/asthma_qrg_0_0.pdf. Accessed May 16, 2023.
- Asthma action plans. Centers for Disease Control and Prevention. https://www.cdc.gov/asthma/actionplan.html. Accessed May 8, 2023.
- Learn More Breathe Better (LLMB): Monitoring your asthma. National Heart, Lung, and Blood Institute. https://www.nhlbi.nih.gov/resources/lmbb-monitoring-your-asthma. Accessed May 16, 2023.
- Asthma: Diagnosis. National Heart, Lung, and Blood Institute. https://www.nhlbi.nih.gov/health/asthma/diagnosis. Accessed May 16, 2023.
- Learn More Breath Better (LLMB): Tips for talking to your health care provider about asthma. National Heart, Lung, and Blood Institute. https://www.nhlbi.nih.gov/resources/lmbb-tips-talking-your-health-care-provider-about-asthma. Accessed May 16, 2023.
Products and Services
- A Book: Mayo Clinic Book of Home Remedies
- Albuterol side effects
- Allergies and asthma
- Allergy shots
- Aspirin allergy
- Asthma and acid reflux
- Asthma attack
- Asthma diet
- Asthma inhalers: Which one's right for you?
- Asthma: Colds and flu
- Asthma medications
- Asthma: Testing and diagnosis
- Cervical cerclage
- Churg-Strauss syndrome
- COVID-19: Who's at higher risk of serious symptoms?
- Exercise and chronic disease
- Exercise-induced asthma
- Intermittent fasting
- Laser eye surgery
- Methacholine challenge test
- Nitric oxide test for asthma
- Occupational asthma
- Ozone air purifiers
- Prednisone risks, benefits
- Prednisone withdrawal: Why taper down slowly?
- Shortness of breath
- Symptom Checker
- Asthma attack video
- Dry powder disk inhaler
- Dry powder tube inhaler
- Video: How to use a peak flow meter
- Single-dose dry powder inhaler
- Using a metered dose asthma inhaler and spacer
- Vocal cord dysfunction
- What is aspirin-exacerbated respiratory disease (AERD)?
Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission.
- Opportunities
Mayo Clinic Press
Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press .
- Mayo Clinic on Incontinence - Mayo Clinic Press Mayo Clinic on Incontinence
- The Essential Diabetes Book - Mayo Clinic Press The Essential Diabetes Book
- Mayo Clinic on Hearing and Balance - Mayo Clinic Press Mayo Clinic on Hearing and Balance
- FREE Mayo Clinic Diet Assessment - Mayo Clinic Press FREE Mayo Clinic Diet Assessment
- Mayo Clinic Health Letter - FREE book - Mayo Clinic Press Mayo Clinic Health Letter - FREE book
- Asthma treatment 3 steps to better asthma control
Make twice the impact
Your gift can go twice as far to advance cancer research and care!
- Open access
- Published: 13 August 2021
Biological therapy for severe asthma
- Silvano Dragonieri ORCID: orcid.org/0000-0003-1563-6864 1 &
- Giovanna Elisiana Carpagnano 1
Asthma Research and Practice volume 7 , Article number: 12 ( 2021 ) Cite this article
12k Accesses
19 Citations
3 Altmetric
Metrics details
Around 5–10% of the total asthmatic population suffer from severe or uncontrolled asthma, which is associated with increased mortality and hospitalization, increased health care burden and worse quality of life. In the last few years, new drugs have been launched and several asthma phenotypes according to definite biomarkers have been identified. In particular, therapy with biologics has revolutionized the management and the treatment of severe asthma, showing high therapeutic efficacy associated with significant clinical benefits. To date, four types of biologics are licensed for severe asthma, i.e. omalizumab (anti-immunoglobulin E) antibody, mepolizumab and reslizumab (anti-interleukin [IL]-5antibody), benralizumab (anti-IL-5 receptor a antibody) and dupilumab (anti-IL-4 receptor alpha antibody). The aim of this article was to review the biologic therapies currently available for the treatment of severe asthma, in order to help physicians to choose the most suitable biologic agent for their asthmatic patients.
Since the beginning of this millennium, asthma assessment and management have been revolutionized. While some new therapeutic approaches have been suggested for mild asthmatics, the most relevant changes have occurred in severe asthma. Severe asthma accounts for the 5–10% of the global asthma population, with 3 to 5% being uncontrolled despite adherence to therapy and proper use of inhalers [ 1 ]. These subjects cannot achieve symptoms control despite maximal therapy with inhaled corticosteroids (ICS) and, quite often, maintenance oral corticosteroids (OCS) are necessary in an endeavor to avoid life-threatening exacerbations [ 2 ]. Although OCS courses remain essential for the management of acute exacerbations, their recurrent or continuous usage is associated with several complications, such as an increased risk of developing osteoporotic fractures and pneumonia [ 3 ]. Moreover, other conditions including cardiovascular and cerebrovascular events, renal dysfunction, diabetes mellitus type 2, humor alterations, obesity and sleep apneas are known to be associated with systemic corticosteroid exposure [ 3 ]. Additionally, many patients remain poorly controlled and show recurrent exacerbations despite a strict adherence to therapy [ 4 ].
The recent advances in our knowledge of the etiopathological mechanisms of different phenotypes and endotypes of severe asthma gave us very innovative therapies, such as biological drugs for severe asthma. These medications are mostly directed against molecules involved in the type 2 inflammatory pathway, thus modifying the natural course of the disease by reducing airways inflammation without the collateral damage associated with corticosteroids. Based on the above, the aim of this article was to review the biologic therapies currently available for the treatment of severe asthma, in order to help physicians to choose the most suitable biologic agent for their asthmatic patients.
Licensed medications for severe asthma
To date, there are five biologic molecules officially approved for use in selected severe asthmatic patients. The first of these is omalizumab, an anti-IgE monoclonal antibody acting through various mechanisms on allergic pathways (Table 1 ). Three more biologics for asthma, belonging to a different class, have been approved, i.e. mepolizumab, reslizumab and benralizumab. They all target the interleukin-5 (IL-5) pathway with the first two targeting the interleukin itself and the last one its receptor. Finally, dupilumab is a monoclonal antibody against the receptor of interleukin-4 (IL-4) which blocks the signaling pathways of IL-4 and IL-13.
BIOLOGICS TARGETING IgE
Omalizumab was the first targeted biologic therapy developed and licensed for severe asthma, being approved by the Food and Drugs Administration in 2003 [ 5 ]. It is a recombinant monoclonal Antibody which binds to IgE, thereby lowering blood IgE levels of up to 99% [ 6 ]. Moreover, It decreases expression of IgE receptor FCRI on inflammatory cells such as mast cells and basophils, thus helping to both mitigate the allergic response and strengthen the antiviral immune response, finally leading to prevent asthma exacerbations [ 7 ]. Omalizumab is approved in adults and children above 6 years old with IgE-driven moderate-to-severe persistent allergic asthma which remains uncontrolled despite GINA step 4/5 treatment, high levels of blood IgE, and documented sensitization to a perennial allergen [ 8 ]. Its dosage varies according to patient’s bodyweight and circulating IgE levels and it is administered subcutaneously every 14 or 28 days [ 9 ]. Although not necessary from a safety point of view, it is advisable to re-evaluate patients after the initial 16 weeks of treatment to assess the drug efficacy before continuing with omalizumab therapy [ 8 ].
The efficacy and safety of omalizumab are nowadays unquestionably recognized, with numerous studies demonstrating that this biological is generally well-tolerated, with no serious adverse effects reported [ 10 , 11 , 12 , 13 , 14 , 15 ]. Common side effects include injection site or diffuse rash, fever, nose bleeding, joint pain, gastro-intestinal disturbances, headache, dizziness and cold symptoms [ 10 , 11 , 12 , 13 , 14 , 15 ]. A Cochrane systematic review assessing 25 randomized controlled trials in patients with allergic asthma showed the efficacy of omalizumab in reducing asthma exacerbations, hospitalizations, and inhaled corticosteroid dosage [ 10 , 15 , 16 , 17 , 18 , 19 ].
During the last few years, a number of biomarkers for monitoring the efficacy of omalizumab therapy have been proposed, including total and antigen-specific IgE, blood eosinophil count and exhaled nitric oxide (FeNO) [ 20 , 21 ]. Surprisingly, total IgE did not appear to be a reliable predictor of response to omalizumab therapy, evidencing that our knowledge on this field is still limited [ 21 ]. Peripheral blood eosinophil count ≥300 cells/mL are linked to higher asthma severity and to a better response to omalizumab [ 22 , 23 ]. Furthermore, patients under omalizumab with higher blood eosinophil count have a higher chance to suffer from asthma exacerbations in case of omalizumab discontinuation [ 24 ]. Regarding FeNO, elevated values at baseline correlated with a better response to omalizumab with regard to exacerbations decrease [ 20 , 25 ]. Likewise, elevated levels of FeNO after suspension of long-term therapy with omalizumab may be a predictor of successive exacerbations [ 24 ].
Biologics targeting IL-5
IL-5 is a well-known regulator of the activation, differentiation, effector function, migration and survival and effector function of eosinophils [ 26 ]. Eosinophil levels associated with symptoms of asthma correlate with disease severity and increase the risk of asthma exacerbations, evidencing that this granulocyte type plays a key role in the pathophysiololgy of asthma [ 26 ]. Currently, licensed biologics against IL-5 pathways are mepolizumab, reslizumab, and benralizumab.
MEPOLIZUMAB
Mepolizumab is a monoclonal antibody directed against IL-5 which has been approved as an add-on treatment for patients ≥6 years old in Europe and for patients ≥12 years old in the USA. Mepolizumab was the first anti-IL-5 antibody approved for the treatment of severe asthma by the Food and Drugs Administration in 2015. Eligible subjects are those with severe eosinophilic asthma that remains uncontrolled despite GINA step 4/5 therapy, with blood eosinophil count of ≥150 cells/μl during the first administration or ≥ 300 cells/μl in the previous year and with at least 2 asthma exacerbations requiring systemic steroid course in the past year [ 27 , 28 ]. Mepolizumab is administered by a subcutaneous injection at a fixed dose of 100 mg every 28 days.
Several studies evaluating mepolizumab for uncontrolled eosinophilic asthma showed a markedly reduction with regard to number of exacerbations, systemic corticosteroid usage, emergency room accesses and hospital admissions, and a concurrent improvement of asthma controls and lung function parameters [ 29 , 30 , 31 , 32 , 33 ].
Furthermore, a number of studies revealed that mepolizumab has a positive long-term safety profile [ 34 , 35 , 36 ]. No reports of mepolizumab-associated anaphylaxis reactions were documented, as well as parasitic infections [ 34 , 35 , 36 ]. Common side effects include headache, injection site reaction, fatigue, flu symptoms, urinary tract infection, abdominal pain, itching, eczema, and muscle spasms [ 34 , 35 , 36 ].
Additionally, numerous investigations highlighted that the most important markers of response prediction to mepolizumab are the rate of previous exacerbation and baseline peripheral blood eosinophil count [ 29 , 32 , 37 , 38 , 39 ]. Indeed, a better clinical efficacy is directly proportional to a higher eosinophil count and to a higher rate of exacerbations [ 29 , 32 , 37 , 38 , 39 ]. Interestingly, mepolizumab effectiveness was not related to baseline IgE and to atopy [ 40 , 41 ] and earlier treatment with omalizumab is not a predictor for mepolizumab efficacy [ 42 , 43 , 44 ].
There is a lack of consensus about the duration of treatment before evaluating the effectiveness of mepolizumab. Actually, the GINA statement suggests that a 4-month trial may be adequate [ 8 ], whereas the NICE guidelines recommend that mepolizumab should not be discontinued before 12 months of therapy and that drug-responsiveness should be assessed every year [ 45 ].
Reslizumab is monoclonal antibody approved in 2016, which binds with high-affinity to IL-5 [ 46 ]. By an analogous mechanism of action to mepolizumab, reslizumab lowers circulating blood eosinophil levels [ 47 ]. It has been approved for patients ≥18 years old with severe eosinophilic asthma which remains uncontrolled despite therapy with high-doses of ICS plus another inhaler. Reslizumab is indicated in patients with ≥400 eosinophils/μl and history of asthma exacerbations in the previous 12 months [ 48 , 49 ]. Reslizumab is administered intravenously every 28 days at a weight-based dose of 3 mg/kg.
Similarly to mepolizumab, studies assessing reslizumab have shown a decreased number of asthma exacerbations and improved asthma control and lung function parameters in subjects with high blood eosinophil levels [ 47 , 50 ].
The safety profile of reslizumab has been evaluated for up to 24 months, revealing minor adverse effects without any reports of parasitic and opportunistic infections [ 51 ]. Most frequent side effects include cough, dizziness, itching, skin rash and fatigue [ 51 ].
However, despite its proven excellent clinical efficacy, intravenous formulation has a significant impact on the ease of administration compared to mepolizumab and/or benralizumab. Studies using reslizumab showed unsatisfactory results, without significant improvements in terms of acute exacerbations reduction or OCS lowering [ 52 ].
BENRALIZUMAB
Benralizumab is a monoclonal antibody approved in 2017 and directed against IL-5 receptor a (IL-5Ra) which induces eosinophil apoptosis via the antibody-dependent cell-mediated cytotoxicity (ADCC) involving natural killer cells, leading to peripheral blood eosinophil depletion [ 53 , 54 ]. Benralizumab acts like a competitive inhibitor to IL-5, binding with higher affinity to the a-subunit of IL-5Ra, which is expressed on mature (and precursors) eosinophils and basophils [ 55 ].
This biologic drug is licensed as an add-on treatment for uncontrolled severe eosinophilic asthma in patients ≥18 years with ≥300 blood eosinophils/μl [ 56 , 57 ]. A 30 mg dose of benralizumab is injected subcutaneously every 28 days for the first 3 administrations and afterwards every 56 days.
Large studies evaluating benralizumab in patients with moderate to severe asthma have shown a decrease in exacerbations number, improved lung function, and reduced use of OCS [ 53 , 54 , 58 ]. Combined analysis of these investigation have revealed that the best predictors of response to benralizumab are adult-onset asthma, more than 3 exacerbations in the previous year, nasal polyposis and pre-bronchodilator FVC < 65% of predicted [ 53 , 54 , 58 ].. The most common adverse effect were fever after the first injection, headache and pharyngitis [ 53 , 54 , 58 ].
Interestingly, based on its mechanism, benralizumab almost completely depletes blood eosinophils within 24 h of administration and a total depletion of airway eosinophils compared to that caused by mepolizumab [ 59 , 60 ]. Likewise, nasal eosinophils were totally suppressed after 6 months of therapy with benralizumab [ 61 ].
Recently, some concerns have been raised about the theoretical risks following an eosinophil depletion, especially with respect to host defense. However, these warnings were not confirmed, since it appears that there is adequate redundancy within human immune apparatus, which is not impaired by eosinophils depletion [ 62 ].
Biologics targeting IL-4 and IL-13
IL-4 and IL-13 are two interleukins which regulate and drive Type-2 inflammation. IL-4 increases the Th-2 cell population and B-cell isotype rearrangement of IgE as well as promoting eosinophilic transmigration through endothelium, whereas IL-13 plays an important role in asthma by promoting airway hyperresponsiveness, mucus secretion and airway remodeling [ 63 , 64 ]. Thus far, the only licensed drug acting on the two aforementioned ILs is dupilumab.
Dupilumab is a monoclonal antibody approved in 2018 which binds to the IL-4 receptor alpha-subunit, mutual to IL-4 and IL-13 receptors and inhibits both IL-4 and IL-13 pathways. Dupilumab is licensed as an add-on maintenance therapy in asthmatic patients GINA step 4/5 ≥ 12 years with type 2 inflammation characterized by increased blood eosinophils and/or raised FeNO. Dupilumab is administered subcutaneously at a starting dose of two injections of 200 mg each (total 400 mg), followed by one injection of 200 mg every 14 days, or at a starting dose of 600 mg (two injections of 300 mg each) followed by 300 mg every 14 days. The latter regimen is recommended for asthmatic subjects strictly dependent from OCS or with atopic dermatitis [ 65 ]. Dupilumab is also indicated for moderate to severe atopic dermatitis and for nasal polyposis.
A number of studies have demonstrated that therapy with dupilumab in severe asthmatics lowers the number of asthma exacerbations, improves lung function parameters and asthma control test scores, and lowers the use of OCS, irrespective of peripheral blood eosinophil count [ 66 , 67 , 68 , 69 ]. Indeed, a transitory increase of blood eosinophilia at the beginning of treatment with dupilumab has been observed although it may be due to blocked migration into tissues rather than hyperproduction [ 69 ]. Furthermore, reduced levels of T2 inflammation markers, including FeNO, serum levels of eotaxin-3, periostin and thymus and activation regulated chemokine (TARC) and total IgE, may serve as parameters for monitoring the efficacy of therapy with dupilumab [ 66 , 67 , 68 , 69 ]. The most common adverse reactions were injection site reactions, various types of infections, conjunctivitis and related conditions [ 66 , 67 , 68 , 69 ].
Biologics under development
Research for next-generation biologics is ongoing. Currently, other effector molecules are under the spotlight as new targets for perspective biological therapies, particularly the so-called alarmins [ 70 ]. These molecules are released by the airway epithelium against the harmful actions of germs, pollutants, allergens and cigarette smoke.
Tezepelumab is a human monoclonal antibody which binds to thymic stromal lymphopoietin (TSLP), an epithelium-derived alarmin that plays a relevant role in the pathogenesis of asthma, being an upstream effector T2-high pathobiologic pathways [ 71 , 72 , 73 ]. With the presence of tezepelumab, TLSP cannot bind to its receptor [ 74 ] hence inhibiting downstream signaling. A number of phase 2 and 3 trials have clearly shown that patients with severe uncontrolled asthma who received tezepelumab had fewer exacerbations and better lung function, asthma control, and health-related quality of life than those who received placebo [ 75 , 76 ]. Concerning its safety profile, neither investigational tezepelumab-related anaphylactic reactions nor the detection of neutralizing antibodies were reported [ 75 , 76 ]. To date, license application for tezepelumab has been accepted and granted Priority Review for the treatment of asthma from the US Food and Drug Administration, whose regulatory decision is expected during the first quarter of 2022.
Ipetekimab is a monoclonal antibody targeting IL-33, another alarmin which associates with TSLP leading to an activation of T2-high inflammatory pathway in asthma [ 77 ]. Phase 2 studies with this biologic are ongoing, however preliminary results did not show adequate efficacy in severe asthmatics when associated with dupilumab or vs dupilumab alone [ 70 ].
Moreover, Tralokinumab and lebrokizumab are monoclonal antibodies both targeting IL-13 alone with disappointing results of phase 3 studies in terms of exacerbations reduction and OCS sparing in severe asthmatics [ 78 ].
Finally, regarding Th2-low asthma, mainly characterized by a neutrophilic airways inflammation, efforts are focusing on its pathogenic cascade involving cytokines such as IL-1beta, IL-17 and IL-23. Several monoclonal antibodies against the aforementioned interleukins such as canakinumab (anti IL-1beta), brodalumab (anti IL-17 receptor) and risankizumab (anti IL-23) are under evaluation with phase 1–2 trials showing controversial results [ 79 , 80 , 81 ].
Which biologic should I choose for my asthmatic patient?
When choosing a biologic medication for their patients with severe uncontrolled asthma, clinicians should always take into account the asthma endotype, clinical biomarkers, and patient-focused aspects (Fig 1 ).
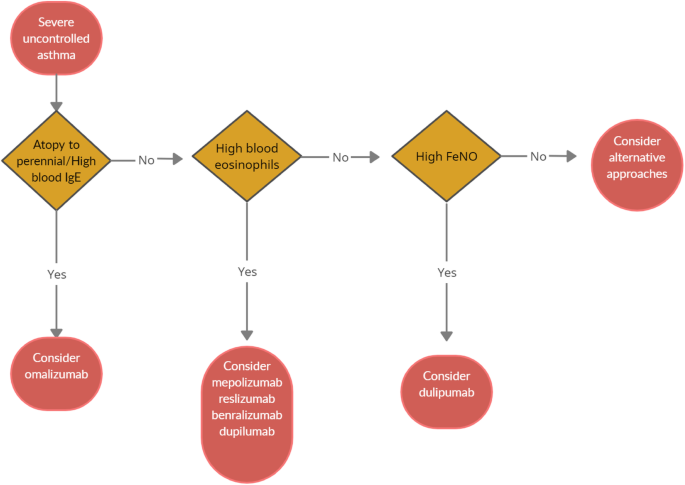
Algorithm for Selecting Ideal Biologic Treatment for severe uncontrolled asthma
Omalizumab should always be the first biological option in allergic non-eosinophilic severe asthmatics, with high levels of blood IgE, and with at least a documented positivity to a perennial aeroallergen. Contrariwise, patients with a non-allergic eosinophilic phenotype should be treated with an anti-IL-5 biological drug. Finally, anti- IL-4/IL-13 should be reserved to patients with severe eosinophilic type 2 asthma OCS dependent [ 8 ].
Given to the a lack of comparison studies, to date there are no recommendations about the selection of appropriate anti IL-5 biologic drug among those available. Hence, the choice is empirical and possibly shared between physician and patient.
According to GINA guidelines, a (at least) 4-month trial should be carried to evaluate asthma control. In the event of poor asthma control, a switch to a different biological treatment can be attempted if the patient meets the eligibility criteria.
Nevertheless, the right time and the right modality of switching from one biologic to another and the treatment time are still unknown. Large studies focused on biological drug switch in patients with severe asthma are ongoing and will help physicians to ease therapeutic strategies.
Conclusions
Severe asthma accounts for a small proportion of total asthma cases, but impose a heavy burden on health care system. Recent revelations of the T2 inflammatory pathways and the development of monoclonal antibodies acting on the T2 cascade has completely revolutionized the management of severe asthma, by introducing new, life-improving treatment options for this class of patients. This paves the way for a biomarker-driven personalized medicine. Strictly following GINA recommendations, the categorization of T2 molecular targets has allowed the identification of patients with severe asthma who would likely respond to specific biological molecules. However, the most suitable biological option for severe asthmatics with overlapping phenotypes is still unclear, thus requiring further discriminatory and predicting biomarkers which may allow a better patient selection.
Availability of data and materials
Not applicable.
Abbreviations
interleukin
inhaled corticosteroids
oral corticosteroids
immunoglobulin E
fractional exhaled nitric oxide
forced vital capacity
Hekking PPW, Wener RR, Amelink M, Zwinderman AH, Bouvy ML, Bel EH. The prevalence of severe refractory asthma. J Allergy Clin Immunol. 2015;135(4):896–902. https://doi.org/10.1016/j.jaci.2014.08.042 .
Article PubMed Google Scholar
Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43(2):343–73. https://doi.org/10.1183/09031936.00202013 .
Article CAS PubMed Google Scholar
Price DB, Trudo F, Voorham J, Xu X, Kerkhof M, Ling Zhi Jie J, et al. Adverse outcomes from initiation of systemic corticosteroids for asthma: long-term observational study. J Asthma Allergy. 2018;11:193–204. https://doi.org/10.2147/JAA.S176026 .
Article CAS PubMed PubMed Central Google Scholar
Sulaiman I, Greene G, MacHale E, Seheult J, Mokoka M, D’Arcy S, et al. A randomised clinical trial of feedback on inhaler adherence and technique in patients with severe uncontrolled asthma. Eur Respir J. 2018;51(1):1701126. https://doi.org/10.1183/13993003.01126-2017 .
Miranda C, Busacker A, Balzar S, Trudeau J, Wenzel SE. Distinguishing severe asthma phenotypes: role of age at onset and eosinophilic inflammation. J Allergy Clin Immunol. 2004;113(1):101–8. https://doi.org/10.1016/j.jaci.2003.10.041 .
Normansell R, Walker S, Milan SJ, Walters EH, Nair P. Omalizumab for asthma in adults and children. Cochrane Database Syst Rev 2014 https://doi.org/10.1002/14651858 . CD003559.pub4.
Teach SJ, Gill MA, Togias A, Sorkness CA, Arbes SJ, Calatroni A, et al. Preseasonal treatment with either omalizumab or an inhaled corticosteroid boost to prevent fall asthma exacerbations. J Allergy Clin Immunol. 2015;136(6):1476–85. https://doi.org/10.1016/j.jaci.2015.09.008 .
Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention. 2021. https://ginasthma.org/ .
European Medicines Agency. EMEA/H/C/000606. 2014. www.ema.europa.eu/en/documents/overview/xolair-epar-summary-public_en.pdf . Accessed 30 May 2021.
Busse W, Corren J, Lanier BQ, McAlary M, Fowler-Taylor A, Cioppa GD, et al. Omalizumab, anti-IgE recombinant humanized monoclonal antibody, for the treatment of severe allergic asthma. J Allergy Clin Immunol. 2001;108(2):184–90. https://doi.org/10.1067/mai.2001.117880 .
Rodrigo GJ, Neffen H, Castro-Rodriguez JA. Efficacy and safety of subcutaneous omalizumab vs placebo as add-on therapy to corticosteroids for children and adults with asthma: a systematic review. Chest. 2011;139:28e35.
Article Google Scholar
Alhossan A, Lee CS, MacDonald K, Abraham I. “Real-life” effectiveness studies of omalizumab in adult patients with severe allergic asthma: meta-analysis. J Allergy Clin Immunol Pract. 2017;5(5):1362–70. https://doi.org/10.1016/j.jaip.2017.02.002 .
Ohta K, Miyamoto T, Amagasaki T, Yamamoto M, Study G. Efficacy and safety of omalizumab in an Asian population with moderate-to-severe persistent asthma. Respirology. 2009;14(8):1156–65. https://doi.org/10.1111/j.1440-1843.2009.01633.x .
Adachi M, Kozawa M, Yoshisue H, Lee Milligan K, Nagasaki M, Sasajima T, et al. Real-world safety and efficacy of omalizumab in patients with severe allergic asthma: a long-term post-marketing study in Japan. Respir Med. 2018;141:56–63. https://doi.org/10.1016/j.rmed.2018.06.021 .
Ledford D, Busse W, Trzaskoma B, Omachi TA, Rosen K, Chipps BE, et al. A randomized multicenter study evaluating Xolair persistence of response after long-term therapy. J Allergy Clin Immunol. 2017;140(1):162–9. https://doi.org/10.1016/j.jaci.2016.08.054 .
Normansell R, Walker S, Milan SJ, Walters EH, Nair P. Omalizumab for asthma in adults and children. Cochrane Database Syst Rev 2014:CD003559.
[Holgate ST, Chuchalin AG, Hebert J, Lotvall J, Persson GB, Chung KF, et al. Efficacy and safety of a recombinant antiimmunoglobulin E antibody (omalizumab) in severe allergic asthma. Clin Exp Allergy 2004;34:632–638.
Soler M, Matz J, Townley R, Buhl R, O’Brien J, Fox H, et al. The anti-IgE antibody omalizumab reduces exacerbations and steroid requirement in allergic asthmatics. Eur Respir J. 2001;18(2):254–61. https://doi.org/10.1183/09031936.01.00092101 .
Busse WW, Morgan WJ, Gergen PJ, Mitchell HE, Gern JE, Liu AH, et al. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. N Engl J Med. 2011;364(11):1005–15. https://doi.org/10.1056/NEJMoa1009705 .
Hanania NA, Wenzel S, Rosen K, Hsieh HJ, Mosesova S, Choy DF, et al. Exploring the effects of omalizumab in allergic asthma: an analysis of biomarkers in the EXTRA study. Am J Respir Crit Care Med. 2013;187(8):804–11. https://doi.org/10.1164/rccm.201208-1414OC .
Tabatabaian F, Ledford DK. Omalizumab for severe asthma: toward personalized treatment based on biomarker profile and clinical history. J Asthma Allergy. 2018;11:53–61. https://doi.org/10.2147/JAA.S107982 .
Casale TB, Chipps BE, Rosen K, Trzaskoma B, Haselkorn T, Omachi TA, et al. Response to omalizumab using patient enrichment criteria from trials of novel biologics in asthma. Allergy. 2018;73(2):490–7. https://doi.org/10.1111/all.13302 .
Busse W, Spector S, Rosen K, Wang Y, Alpan O. High eosinophil count: a potential biomarker for assessing successful omalizumab treatment effects. J Allergy Clin Immunol. 2013;132(2):485–6. https://doi.org/10.1016/j.jaci.2013.02.032 .
Ledford D, Busse W, Trzaskoma B, Omachi TA, Rosen K, Chipps BE, et al. A randomized multicenter study evaluating Xolair persistence of response after longterm therapy. J Allergy Clin Immunol. 2017;140(1):162–9. https://doi.org/10.1016/j.jaci.2016.08.054 .
Mansur AH, Srivastava S, Mitchell V, Sullivan J, Kasujee I. Longterm clinical outcomes of omalizumab therapy in severe allergic asthma: study of efficacy and safety. Respir Med. 2017;124:36–43. https://doi.org/10.1016/j.rmed.2017.01.008 .
Akdis CA, Arkwright PD, Bruggen MC, Busse W, Gadina M, Guttman-Yassky E, et al. Type 2 immunity in the skin and lungs. Allergy. 2020;75(7):1582–605. https://doi.org/10.1111/all.14318 .
US Food and Drug Administration. NUCALA (mepolizumab) for injection, for subcutaneoususe.2015. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/125526s004lbl.pdf . .
European Medicines Agency. Nucala. EMEA/H/C/003860-N/0027. 2015. https://www.ema.europa.eu/en/documents/product-information/nucala-eparproduct-information_en.pdf . Accessed 1 Jun 2021.
Ortega HG, Liu MC, Pavord ID, Brusselle GG, FitzGerald JM, Chetta A, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371(13):1198–207. https://doi.org/10.1056/NEJMoa1403290 .
Haldar P, Brightling CE, Hargadon B, Gupta S, Monteiro W, Sousa A, et al. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med. 2009;360(10):973–84. https://doi.org/10.1056/NEJMoa0808991 .
Nair P, Pizzichini MM, Kjarsgaard M, Inman MD, Efthimiadis A, Pizzichini E, et al. Mepolizumab for prednisone-dependent asthma with sputum eosinophilia. N Engl J Med. 2009;360(10):985–93. https://doi.org/10.1056/NEJMoa0805435 .
Pavord ID, Korn S, Howarth P, Bleecker ER, Buhl R, Keene ON, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet. 2012;380(9842):651–9. https://doi.org/10.1016/S0140-6736(12)60988-X .
Bel EH, Wenzel SE, Thompson PJ, Prazma CM, Keene ON, Yancey SW, et al. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014;371(13):1189–97. https://doi.org/10.1056/NEJMoa1403291 .
Lugogo N, Domingo C, Chanez P, Leigh R, Gilson MJ, Price RG, et al. Long-term efficacy and safety of mepolizumab in patients with severe eosinophilic asthma: a multi-center, open-label, phase IIIb study. Clin Ther. 2016;38(9):2058–70. https://doi.org/10.1016/j.clinthera.2016.07.010 .
Khatri S, Moore W, Gibson PG, Leigh R, Bourdin A, Maspero J, et al. Assessment of the long-term safety of mepolizumab and durability of clinical response in patients with severe eosinophilic asthma. J Allergy Clin Immunol. 2018;143:1742–51.
Khurana S, Brusselle GG, Bel EH, FitzGerald JM, Masoli M, Korn S, et al. Long-term safety and clinical benefit of mepolizumab in patients with the most severe eosinophilic asthma: the COSMEX study. Clin Ther. 2019;41(10):2041–56. https://doi.org/10.1016/j.clinthera.2019.07.007 .
Ortega HG, Yancey SW, Mayer B, Gunsoy NB, Keene ON, Bleecker ER, et al. Severe eosinophilic asthma treated with mepolizumab stratified by baseline eosinophil thresholds: a secondary analysis of the DREAM and MENSA studies. Lancet Respir Med. 2016;4(7):549–56. https://doi.org/10.1016/S2213-2600(16)30031-5 .
Ortega H, Li H, Suruki R, Albers F, Gordon D, Yancey S: Cluster analysis and characterization of response to mepolizumab. A step closer to personalized medicine for patients with severe asthma. Ann Am Thorac Soc 2014;11:1011–1017, 7, DOI: https://doi.org/10.1513/AnnalsATS.201312-454OC .
Katz LE, Gleich GJ, Hartley BF, Yancey SW, Ortega HG. Blood eosinophil count is a useful biomarker to identify patients with severe eosinophilic asthma. Ann Am Thorac Soc. 2014;11(4):531–6. https://doi.org/10.1513/AnnalsATS.201310-354OC .
Ortega H, Chupp G, Bardin P, Bourdin A, Garcia G, Hartley B, et al. The role of mepolizumab in atopic and nonatopic severe asthma with persistent eosinophilia. Eur Respir J. 2014;44(1):239–41. https://doi.org/10.1183/09031936.00220413 .
Prazma CM, Wenzel S, Barnes N, Douglass JA, Hartley BF, Ortega H. Characterisation of an OCS-dependent severe asthma population treated with mepolizumab. Thorax. 2014;69(12):1141–2. https://doi.org/10.1136/thoraxjnl-2014-205581 .
Magnan A, Bourdin A, Prazma CM, Albers FC, Price RG, Yancey SW, et al. Treatment response with mepolizumab in severe eosinophilic asthma patients with previous omalizumab treatment. Allergy. 2016;71(9):1335–44. https://doi.org/10.1111/all.12914 .
Galkin D, Liu MC, Chipps BE, Chapman KR, Munoz X, Angel Bergna M, et al. Efficacy and safety of mepolizumab in uncontrolled patients with severe eosinophilic asthma following a switch from omalizumab (OSMO Study): exacerbation and safety outcomes. J Allergy Clin Immunol. 2018;141(2):AB409. https://doi.org/10.1016/j.jaci.2017.12.965 .
Chapman KR, Albers FC, Chipps B, Munoz X, Devouassoux G, Bergna M, et al. The clinical benefit of mepolizumab replacing omalizumab in uncontrolled severe eosinophilic asthma. Allergy Eur J Allergy Clin Immunol. 2019;74(9):1716–26. https://doi.org/10.1111/all.13850 .
Article CAS Google Scholar
National Institute for Health and Care Excellence (NICE). Mepolizumab for treating severe refractory eosinophilic asthma. 2017. http://www.nice.org.uk/guidance/ta431 . Accessed 1 Jun 2021.
Egan R, Athwal D, Bodmer M, Carter J, Chapman R, Choua CC, et al. Effect of Sch 55700, a humanized monoclonal antibody to human interleukin-5, on eosinophilic responses and bronchial hyperreactivity. Arzneimittelforschung. 2011;49:779–90.
Corren J, Weinstein S, Janka L, Zangrilli J, Garin M. Phase 3 study of reslizumab in patients with poorly controlled asthma. Chest. 2016;150(4):799–810. https://doi.org/10.1016/j.chest.2016.03.018 .
US Food and Drug Administration. CINQAIR (reslizumab) injection, for intravenous use. ReferenceID:3906489.2016. www.accessdata.fda.gov/drugsatfda_docs/label/2016/761033lbl.pdf . .
European Medicines Agency. EMEA/H/C/003912.2016. www.ema.europa.eu/en/documents/overview/cinqaero-epar-summarypublic_en.pdf . Accessed 3 Jun 2021.
Castro M, Zangrilli J, Wechsler ME, Bateman ED, Brusselle GG, Bardin P, et al. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respir Med. 2015;3(5):355–66. https://doi.org/10.1016/S2213-2600(15)00042-9 .
Murphy K, Jacobs J, Bjermer L, Fahrenholz JM, Shalit Y, Garin M, et al. Long-term safety and efficacy of reslizumab in patients with eosinophilic asthma. J Allergy Clin Immunol. 2017;5:1572–81.
Bernstein JA, Virchow JC, Murphy K, Maspero JF, Jacobs J, Adir Y, et al. Effect of fixed-dose subcutaneous reslizumab on asthma exacerbations in patients with severe uncontrolled asthma and corticosteroid sparing in patients with oral corticosteroid- dependent asthma: results from two phase 3, randomised, double-blind, placebo. Lancet Respir Med. 2020;8(5):461–74. https://doi.org/10.1016/S2213-2600(19)30372-8 .
FitzGerald JM, Bleecker ER, Nair P, Korn S, Ohta K, Lommatzsch M, et al. Benralizumab, an anti-interleukin-5 receptor alpha monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2016;388(10056):2128–41. https://doi.org/10.1016/S0140-6736(16)31322-8 .
Nair P, Wenzel S, Rabe KF, Bourdin A, Lugogo NL, Kuna P, et al. Oral glucocorticoid-sparing effect of benralizumab in severe asthma. N Engl J Med. 2017;376(25):2448–58. https://doi.org/10.1056/NEJMoa1703501 .
Ghazi A, Trikha A, Calhoun WJ. Benralizumab – a humanized mAb to IL-5Ra with enhanced antibody-dependent cell-mediated cytotoxicity – a novel approach for the treatment of asthma. Expert Opin Biol Ther. 2012;12(1):113–8. https://doi.org/10.1517/14712598.2012.642359 .
US Food and Drug Administration. FASENRA (benralizumab) injection, for subcutaneous use. ReferenceID:4181236.2019. www.accessdata.fda.gov/drugsatfda_docs/label/2017/761070s000lbl.pdf . .
European Medicines Agency. EMEA/H/C/4433. 2019. www.ema.europa.eu/en/documents/overview/fasenra-epar-medicineoverview_en.pdf . Accessed 3 Jun 2021.
Bleecker ER, FitzGerald JM, Chanez P, Papi A, Weinstein SF, Barker P, et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting beta2-agonists (SIROCCO): a randomised, multicentre, placebo-controlled phase 3 trial. Lancet. 2016;388(10056):2115–27. https://doi.org/10.1016/S0140-6736(16)31324-1 .
Laviolette M, Gossage DL, Gauvreau G, Leigh R, Olivenstein R, Katial R, et al. Effects of benralizumab on airway eosinophils in asthmatic patients with sputum eosinophilia. J Allergy Clin Immunol. 2013;132(5):1086–96. https://doi.org/10.1016/j.jaci.2013.05.020 .
Roxas C, Fernandes M, Green L, D’Ancona G, Kavanagh J, Kent B, et al. A comparison of the clinical response to mepolizumab and benralizumab at 4 weeks. Thorax. 2018;73:A50.
Google Scholar
Buonamico E, Dragonieri S, Sciancalepore PI, Carratù P, Carpagnano GE, Resta O, et al. Assessment of eosinophilic nasal inflammation in patients with severe asthma and nasal polyposis before and after six months of therapy with Benralizumab. J Biol Regul Homeost Agents. 2020;34(6):2353–7. https://doi.org/10.23812/20-323-L .
Jackson DJ, Korn S, Mathur SK, Barker P, Meka VG, Martin UJ, et al. Safety of eosinophil-depleting therapy for severe, eosinophilic asthma: focus on benralizumab. Drug Saf. 2020;43(5):409–25. https://doi.org/10.1007/s40264-020-00926-3 .
Lambrecht BN, Hammad H, Fahy JV. The cytokines of asthma. Immunity. 2019;50(4):975–91. https://doi.org/10.1016/j.immuni.2019.03.018 .
Wynn TA. Type 2 cytokines: mechanisms and therapeutic strategies. Nat Rev Immunol. 2015;15(5):271–82. https://doi.org/10.1038/nri3831 .
European Medicines Agency. Dupinex: EMEA/H/C/004390. 2018. https://www.ema.europa.eu/en/documents/product-information/dupixent-epar-productinformation_en.pdf . Accessed 4 Jun 2021.
Wenzel S, Castro M, Corren J, Maspero J, Wang L, Zhang B, et al. Dupilumab efficacy and safety in adults with uncontrolled persistent asthma despite use of medium-tohigh-dose inhaled corticosteroids plus a long-acting beta2 agonist: a randomised double-blind placebo-controlled pivotal phase 2b dose-ranging trial. Lancet. 2016;388(10039):31–44. https://doi.org/10.1016/S0140-6736(16)30307-5 .
Castro M, Corren J, Pavord ID, Maspero J, Wenzel S, Rabe KF, et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N Engl J Med. 2018;378(26):2486–96. https://doi.org/10.1056/NEJMoa1804092 .
Rabe KF, Nair P, Brusselle G, Maspero JF, Castro M, Sher L, et al. Efficacy and safety of dupilumab in glucocorticoid dependent severe asthma. N Engl J Med. 2018;378(26):2475–85. https://doi.org/10.1056/NEJMoa1804093 .
Huang J, Pansare M. New treatments for asthma. Pediatr Clin. 2019;66(5):925–39. https://doi.org/10.1016/j.pcl.2019.06.001 .
Porsbjerg CM, Sverrild A, Lloyd CM, Menzies-Gow AN, Bel EH. Anti-alarmins in asthma: targeting the airway epithelium with next-generation biologics. Eur Respir J. 2020;56(5):2000260. https://doi.org/10.1183/13993003.00260-2020 .
Harada M, Hirota T, Jodo AI, Hitomi Y, Sakashita M, Tsunoda T, et al. Thymic stromal lymphopoietin gene promoter polymorphisms are associated with susceptibility to bronchial asthma. Am J Respir Cell Mol Biol. 2011;44(6):787–93. https://doi.org/10.1165/rcmb.2009-0418OC .
Li Y, Wang W, Lv Z, Li Y, Chen Y, Huang K, et al. Elevated expression of IL-33 and TSLP in the airways of human asthmatics in vivo: a potential biomarker of severe refractory disease. J Immunol. 2018;200(7):2253–62. https://doi.org/10.4049/jimmunol.1701455 .
He JQ, Hallstrand TS, Knight D, Chan-Yeung M, Sandford A, Tripp B, et al. A thymic stromal lymphopoietin gene variant is associated with asthma and airway hyperresponsiveness. J Allergy Clin Immunol. 2009;124(2):222–9. https://doi.org/10.1016/j.jaci.2009.04.018 .
Verstraete K, Peelman F, Braun H, Lopez J, Van Rompaey D, Dansercoer A, et al. Structure and antagonism of the receptor complex mediated by human TSLP in allergy and asthma. NatCommun. 2017;8:14937.
CAS Google Scholar
Corren J, Parnes JR, Wang L, Mo M, Roseti SL, Griffiths JM, et al. Tezepelumab in adults with uncontrolled asthma. N Engl J Med. 2017;377(10):936–46. https://doi.org/10.1056/NEJMoa1704064 .
Menzies-Gow A, Corren J, Bourdin A, Chupp G, Israel E, Wechsler ME, et al. Tezepelumab in adults and adolescents with severe, uncontrolled asthma. N Engl J Med. 2021;384(19):1800–9. https://doi.org/10.1056/NEJMoa2034975 .
Murakami-Satsutani N, Ito T, Nakanishi T, Inagaki N, Tanaka A, Vien PTX, et al. IL-33 promotes the induction and maintenance of Th2 immune responses by enhancing the function of OX40 ligand. Allergol Int. 2014;63(3):443–55. https://doi.org/10.2332/allergolint.13-OA-0672 .
Busse WW, Brusselle GG, Korn S, Kuna P, Magnan A, Cohen D, et al. Tralokinumab did not demonstrate oral corticosteroid-sparing effects in severe asthma. Eur Respir J. 2019;53(2):1800948. https://doi.org/10.1183/13993003.00948-2018 .
Nair P, Prabhavalkar KS. Neutrophilic asthma and potentially related target therapies. Curr Drug Targets. 2020;21(4):374–88. https://doi.org/10.2174/1389450120666191011162526 .
Kalchiem-Dekel O, Yao X, Levine SJ. Meeting the Challenge of Identifying New Treatments for Type 2-Low Neutrophilic Asthma. Chest;15:26–33.
Busse WW, Holgate S, Kerwin E, Chon Y, Feng J, Lin J, et al. Randomized, double-blind, placebo-controlled study of brodalumab, a human anti-IL-17 receptor monoclonal antibody, in moderate to severe asthma. Am J Respir Crit Care Med. 2013;188(11):1294–302. https://doi.org/10.1164/rccm.201212-2318OC .
Download references
Acknowledgements
Authors did not receive any funding for the current review.
Author information
Authors and affiliations.
Department of Respiratory Diseases, University of Bari “Aldo Moro”, Piazza Giulio Cesare 11, 70124, Bari, Italy
Silvano Dragonieri & Giovanna Elisiana Carpagnano
You can also search for this author in PubMed Google Scholar
Contributions
SD and GEC equally contributed in writing the current review. The author(s) read and approved the final manuscript.
Corresponding author
Correspondence to Silvano Dragonieri .
Ethics declarations
Ethics approval and consent to participate.
Not required.
Consent for publication
Obtained from all authors.

Competing interests
None of the authors have conflicts to disclose.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Dragonieri, S., Carpagnano, G.E. Biological therapy for severe asthma. asthma res and pract 7 , 12 (2021). https://doi.org/10.1186/s40733-021-00078-w
Download citation
Received : 29 June 2021
Accepted : 02 August 2021
Published : 13 August 2021
DOI : https://doi.org/10.1186/s40733-021-00078-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Severe asthma
- Biological therapy
Asthma Research and Practice
ISSN: 2054-7064
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
- Open access
- Published: 15 August 2020
Treatment strategies for asthma: reshaping the concept of asthma management
- Alberto Papi 1 , 7 ,
- Francesco Blasi 2 , 3 ,
- Giorgio Walter Canonica 4 ,
- Luca Morandi 1 , 7 ,
- Luca Richeldi 5 &
- Andrea Rossi 6
Allergy, Asthma & Clinical Immunology volume 16 , Article number: 75 ( 2020 ) Cite this article
46k Accesses
54 Citations
4 Altmetric
Metrics details
Asthma is a common chronic disease characterized by episodic or persistent respiratory symptoms and airflow limitation. Asthma treatment is based on a stepwise and control-based approach that involves an iterative cycle of assessment, adjustment of the treatment and review of the response aimed to minimize symptom burden and risk of exacerbations. Anti-inflammatory treatment is the mainstay of asthma management. In this review we will discuss the rationale and barriers to the treatment of asthma that may result in poor outcomes. The benefits of currently available treatments and the possible strategies to overcome the barriers that limit the achievement of asthma control in real-life conditions and how these led to the GINA 2019 guidelines for asthma treatment and prevention will also be discussed.
Asthma, a major global health problem affecting as many as 235 million people worldwide [ 1 ], is a common, non-communicable, and variable chronic disease that can result in episodic or persistent respiratory symptoms (e.g. shortness of breath, wheezing, chest tightness, cough) and airflow limitation, the latter being due to bronchoconstriction, airway wall thickening, and increased mucus.
The pathophysiology of the disease is complex and heterogeneous, involving various host-environment interactions occurring at various scales, from genes to organ [ 2 ].
Asthma is a chronic disease requiring ongoing and comprehensive treatment aimed to reduce the symptom burden (i.e. good symptom control while maintaining normal activity levels), and minimize the risk of adverse events such as exacerbations, fixed airflow limitation and treatment side effects [ 3 , 4 ].
Asthma treatment is based on a stepwise approach. The management of the patient is control-based; that is, it involves an iterative cycle of assessment (e.g. symptoms, risk factors, etc.), adjustment of treatment (i.e. pharmacological, non-pharmacological and treatment of modifiable risk factors) and review of the response (e.g. symptoms, side effects, exacerbations, etc.). Patients’ preferences should be taken into account and effective asthma management should be the result of a partnership between the health care provider and the person with asthma, particularly when considering that patients and clinicians might aim for different goals [ 4 ].
This review will discuss the rationale and barriers to the treatment of asthma, that may result in poor patient outcomes. The benefits of currently available treatments and the possible strategies to overcome the barriers that limit the achievement of asthma control in real-life situations will also be discussed.
The treatment of asthma: where are we? Evolution of a concept
Asthma control medications reduce airway inflammation and help to prevent asthma symptoms; among these, inhaled corticosteroids (ICS) are the mainstay in the treatment of asthma, whereas quick-relief (reliever) or rescue medicines quickly ease symptoms that may arise acutely. Among these, short-acting beta-agonists (SABAs) rapidly reduce airway bronchoconstriction (causing relaxation of airway smooth muscles).
National and international guidelines have recommended SABAs as first-line treatment for patients with mild asthma, since the Global Initiative for Asthma guidelines (GINA) were first published in 1995, adopting an approach aimed to control the symptoms rather than the underlying condition; a SABA has been the recommended rescue medication for rapid symptom relief. This approach stems from the dated idea that asthma symptoms are related to bronchial smooth muscle contraction (bronchoconstriction) rather than a condition concomitantly caused by airway inflammation. In 2019, the GINA guidelines review (GINA 2019) [ 4 ] introduced substantial changes overcoming some of the limitations and “weaknesses” of the previously proposed stepwise approach to adjusting asthma treatment for individual patients. The concept of an anti-inflammatory reliever has been adopted at all degrees of severity as a crucial component in the management of the disease, increasing the efficacy of the treatment while lowering SABA risks associated with patients’ tendency to rely or over-rely on the as-needed medication.
Until 2017, the GINA strategy proposed a pharmacological approach based on a controller treatment (an anti-inflammatory, the pillar of asthma treatment), with a SABA as an additional rescue intervention. The reliever, a short-acting bronc hodilator, was merely an addendum , a medication to be used in case the real treatment (the controller) failed to maintain disease control: SABAs effectively induce rapid symptom relief but are ineffective on the underlying inflammatory process. Based on the requirement to achieve control, the intensity of the controller treatment was related to the severity of the disease, varying from low-dose ICS to combination low-dose ICS/long-acting beta-agonist (LABA), medium-dose ICS/LABA, up to high-dose ICS/LABA, as preferred controller choice, with a SABA as the rescue medication. As a result, milder patients were left without any anti-inflammatory treatment and could only rely on SABA rescue treatment.
Poor adherence to therapy is a major limitation of a treatment strategy based on the early introduction of the regular use of controller therapy [ 5 ]. Indeed, a number of surveys have highlighted a common pattern in the use of inhaled medication [ 6 ], in which treatment is administered only when asthma symptoms occur; in the absence of symptoms, treatment is avoided as patients perceive it as unnecessary. When symptoms worsen, patients prefer to use reliever therapies, which may result in the overuse of SABAs [ 7 ]. Indirect evidence suggests that the overuse of beta-agonists alone is associated with increased risk of death from asthma [ 8 ].
In patients with mild persistent disease, low-dose ICS decreases the risk of severe exacerbations leading to hospitalization and improves asthma control [ 9 ]. When low-dose ICS are ineffective in controlling the disease (Step 3 of the stepwise approach), a combination of low-dose ICS with LABA maintenance was the recommended first-choice treatment, plus as-needed SABA [ 3 , 10 ]. Alternatively, the combination low-dose ICS/LABA (formoterol) was to be used as single maintenance and reliever treatment (SMART). The SMART strategy containing the rapid-acting formoterol was recommended throughout GINA Steps 3 to 5 based on solid clinical-data evidence [ 3 ].
The addition of a LABA to ICS treatment reduces both severe and mild asthma exacerbation rates, as shown in the one-year, randomized, double-blind, parallel-group FACET study [ 11 ]. This study focused on patients with persistent asthma symptoms despite receiving ICS and investigated the efficacy of the addition of formoterol to two dose levels of budesonide (100 and 400 µg bid ) in decreasing the incidence of both severe and mild asthma exacerbations. Adding formoterol decreased the incidence of both severe and mild asthma exacerbations, independent of ICS dose. Severe and mild exacerbation rates were reduced by 26% and 40%, respectively, with the addition of formoterol to the lower dose of budesonide; the corresponding reductions were 63% and 62%, respectively, when formoterol was added to budesonide at the higher dose.
The efficacy of the ICS/LABA combination was confirmed in the post hoc analysis of the FACET study, in which patients were exposed to a combination of formoterol and low-dose budesonide [ 12 ]. However, such high levels of asthma control are not achieved in real life [ 5 ]. An explanation for this is that asthma is a variable condition and this variability might include the exposure of patients to factors which may cause a transient steroid insensitivity in the inflammatory process. This, in turn, may lead to an uncontrolled inflammatory response and to exacerbations, despite optimal controller treatment. A typical example of this mechanism is given by viral infections, the most frequent triggers of asthma exacerbations. Rhinoviruses, the most common viruses found in patients with asthma exacerbations, interfere with the mechanism of action of corticosteroids making the anti-inflammatory treatment transiently ineffective. A transient increase in the anti-inflammatory dose would overcome the trigger-induced anti-inflammatory resistance, avoiding uncontrolled inflammation leading to an exacerbation episode [ 13 , 14 , 15 ].
Indeed, symptoms are associated with worsening inflammation and not only with bronchoconstriction. Romagnoli et al. showed that inflammation, as evidenced by sputum eosinophilia and eosinophilic markers, is associated with symptomatic asthma [ 16 ]. A transient escalation of the ICS dose would prevent loss of control over inflammation and decrease the risk of progression toward an acute episode. In real life, when experiencing a deterioration of asthma control, patients self-treat by substantially increasing their SABA medication (Fig. 1 ); it is only subsequently that they (modestly) increase the maintenance treatment [ 17 ].
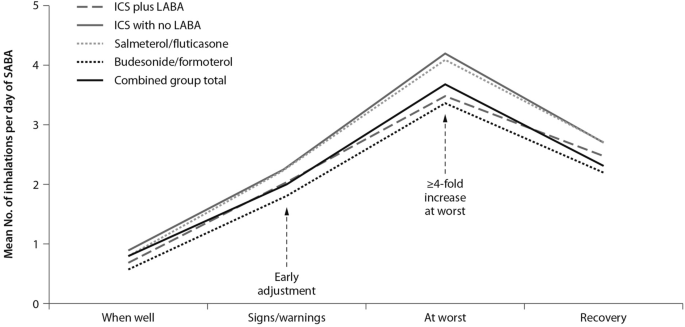
Mean use of SABA at different stages of asthma worsening. Patients have been grouped according to maintenance therapy shown in the legend. From [ 17 ], modified
As bronchodilators, SABAs do not control the underlying inflammation associated with increased symptoms. The “as required” use of SABAs is not the most effective therapeutic option in controlling a worsening of inflammation, as signaled by the occurrence of symptoms; instead, an anti-inflammatory therapy included in the rescue medication along with a rapid-acting bronchodilator could provide both rapid symptom relief and control over the underlying inflammation. Thus, there is a need for a paradigm shift, a new therapeutic approach based on the rescue use of an inhaled rapid-acting beta-agonist combined with an ICS: an anti-inflammatory reliever strategy [ 18 ].
The symptoms of an exacerbation episode, as reported by Tattersfield and colleagues in their extension of the FACET study, increase gradually before the peak of the exacerbation (Fig. 2 ); and the best marker of worsening asthma is the increased use of rescue beta-agonist treatment that follows exactly the pattern of worsening symptomatology [ 19 ]. When an ICS is administered with the rescue bronchodilator, the patient would receive anti-inflammatory therapy when it is required; that is, when the inflammation is uncontrolled, thus increasing the efficiency of the anti-inflammatory treatment.
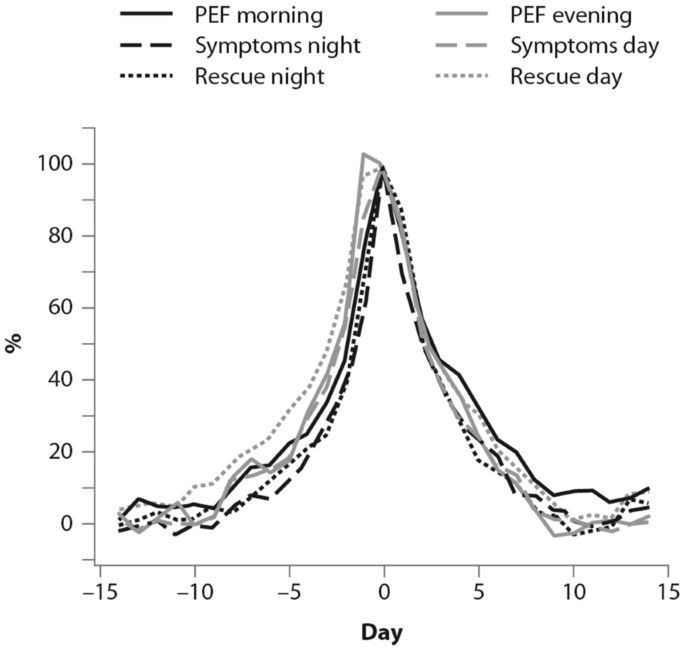
(From [ 19 ])
Percent variation in symptoms, rescue beta-agonist use and peak expiratory flow (PEF) during an exacerbation. In order to allow comparison over time, data have been standardized (Day-14 = 0%; maximum change = 100%)
Barriers and paradoxes of asthma management
A number of barriers and controversies in the pharmacological treatment of asthma have prevented the achievement of effective disease management [ 20 ]. O’Byrne and colleagues described several such controversies in a commentary published in 2017, including: (1) the recommendation in Step 1 of earlier guidelines for SABA bronchodilator use alone, despite asthma being a chronic inflammatory condition; and (2) the autonomy given to patients over perception of need and disease control at Step 1, as opposed to the recommendation of a fixed-dose approach with treatment-step increase, regardless of the level of symptoms [ 20 ]. Other controversies outlined were: (3) a difficulty for patients in understanding the recommendation to minimize SABA use at Step 2 and switch to a fixed-dose ICS regimen, when they perceive SABA use as more effective; (4) apparent conflicting safety messages within the guidelines that patient-administered SABA monotherapy is safe, but patient-administered LABA monotherapy is not; and (5) a discrepancy as to patients’ understanding of “controlled asthma” and their symptom frequency, impact and severity [ 20 ].
Controversies (1) and (2) can both establish an early over-dependence on SABAs. Indeed, asthma patients freely use (and possibly overuse) SABAs as rescue medication. UK registry data have recently suggested SABA overuse or overreliance may be linked to asthma-related deaths: among 165 patients on short-acting relievers at the time of death, 56%, 39%, and 4% had been prescribed > 6, > 12, and > 50 SABA inhalers respectively in the previous year [ 21 ]. Registry studies have shown the number of SABA canisters used per year to be directly related to the risk of death in patients with asthma. Conversely, the number of ICS canisters used per year is inversely related to the rate of death from asthma, when compared with non-users of ICS [ 8 , 22 ]. Furthermore, low-dose ICS used regularly are associated with a decreased risk of asthma death, with discontinuation of these agents possibly detrimental [ 22 ].
Other barriers to asthma pharmacotherapy have included the suggestion that prolonged treatment with LABAs may mask airway inflammation or promote tolerance to their effects. Investigating this, Pauwels and colleagues found that in patients with asthma symptoms that were persistent despite taking inhaled glucocorticoids, the addition of regular treatment with formoterol to budesonide for a 12-month period did not decrease asthma control, and improved asthma symptoms and lung function [ 11 ].
Treatment strategies across all levels of asthma severity
Focusing on risk reduction, the 2014 update of the GINA guidelines recommended as-needed SABA for Step 1 of the stepwise treatment approach, with low-dose ICS maintenance therapy as an alternative approach for long-term anti-inflammatory treatment [ 23 ]. Such a strategy was only supported by the evidence from a post hoc efficacy analysis of the START study in patients with recently diagnosed mild asthma [ 24 ]. The authors showed that low-dose budesonide reduced the decline of lung-function over 3 years and consistently reduced severe exacerbations, regardless of symptom frequency at baseline, even in subjects with symptoms below the then-threshold of eligibility for ICS [ 24 ]. However, as for all post hoc analyses, the study by Reddel and colleagues does not provide conclusive evidence and, even so, their results could have questionable clinical significance for the management of patients with early mild asthma. To be effective, this approach would require patients to be compliant to regular twice-daily ICS for 10 years to have the number of exacerbations reduce by one. In real life, it is highly unlikely that patients with mild asthma would adhere to such a regular regimen [ 25 ].
The 2016 update to the GINA guidelines lowered the threshold for the use of low-dose ICS (GINA Step 2) to two episodes of asthma symptoms per month (in the absence of any supportive evidence for the previous cut-off). The objective was to effectively increase the asthma population eligible to receive regular ICS treatment and reduce the population treated with a SABA only, given the lack of robust evidence of the latter’s efficacy and safety and the fact that asthma is a variable condition characterized by acute exacerbations [ 26 ]. Similarly, UK authorities recommended low-dose ICS treatment in mild asthma, even for patients with suspected asthma, rather than treatment with a SABA alone [ 10 ]. However, these patients are unlikely to have good adherence to the regular use of an ICS. It is well known that poor adherence to treatment is a major problem in asthma management, even for patients with severe asthma. In their prospective study of 2004, Krishnan and colleagues evaluated the adherence to ICS and oral corticosteroids (OCS) in a cohort of patients hospitalized for asthma exacerbations [ 27 ]. The trend in the data showed that adherence to ICS and OCS treatment in patients dropped rapidly to reach nearly 50% within 7 days of hospital discharge, with the rate of OCS discontinuation per day nearly double the rate of ICS discontinuation per day (− 5.2% vs. − 2.7%; p < 0.0001 respectively, Fig. 3 ), thus showing that even after a severe event, patients’ adherence to treatment is suboptimal [ 27 ].

(From [ 27 ])
Use of inhaled (ICS) and oral (OCS) corticosteroids in patients after hospital discharge among high-risk adult patients with asthma. The corticosteroid use was monitored electronically. Error bars represent the standard errors of the measured ICS and OCS use
Guidelines set criteria with the aim of achieving optimal control of asthma; however, the attitude of patients towards asthma management is suboptimal. Partridge and colleagues were the first in 2006 to evaluate the level of asthma control and the attitude of patients towards asthma management. Patients self-managed their condition using their medication as and when they felt the need, and adjusted their treatment by increasing their intake of SABA, aiming for an immediate relief from symptoms [ 17 ]. The authors concluded that the adoption of a patient-centered approach in asthma management could be advantageous to improve asthma control.
The concomitant administration of an as-needed bronchodilator and ICS would provide rapid relief while administering anti-inflammatory therapy. This concept is not new: in the maintenance and reliever approach, patients are treated with ICS/formoterol (fast-acting, long-acting bronchodilator) combinations for both maintenance and reliever therapy. An effective example of this therapeutic approach is provided in the SMILE study in which symptomatic patients with moderate to severe asthma and treated with budesonide/formoterol as maintenance therapy were exposed to three different as-needed options: SABA (terbutaline), rapid-onset LABA (formoterol) and a combination of LABA and ICS (budesonide/formoterol) [ 28 ]. When compared with formoterol, budesonide/formoterol as reliever therapy significantly reduced the risk of severe exacerbations, indicating the efficacy of ICS as rescue medication and the importance of the as-needed use of the anti-inflammatory reliever.
The combination of an ICS and a LABA (budesonide/formoterol) in one inhaler for both maintenance and reliever therapy is even more effective than higher doses of maintenance ICS and LABA, as evidenced by Kuna and colleagues and Bousquet and colleagues (Fig. 4 ) [ 29 , 30 ].
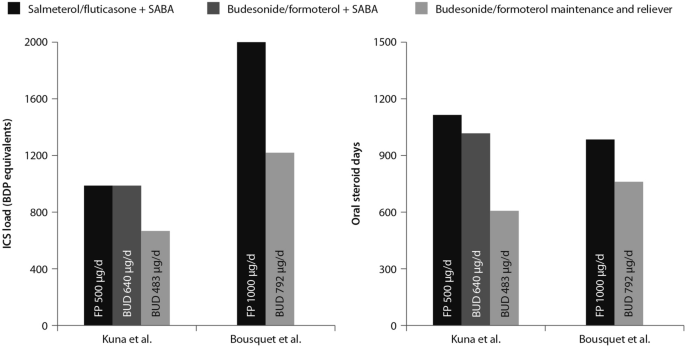
(Data from [ 29 , 30 ])
Comparison between the improvements in daily asthma control resulting from the use of budesonide/formoterol maintenance and reliever therapy vs. higher dose of ICS/LABA + SABAZ and steroid load for the two regimens
The effects of single maintenance and reliever therapy versus ICS with or without LABA (controller therapy) and SABA (reliever therapy) have been recently addressed in the meta-analysis by Sobieraj and colleagues, who analysed 16 randomized clinical trials involving patients with persistent asthma [ 31 ]. The systematic review supported the use of single maintenance and reliever therapy, which reduces the risk of exacerbations requiring systemic corticosteroids and/or hospitalization when compared with various strategies using SABA as rescue medication [ 31 ].
This concept was applied to mild asthma by the BEST study group, who were the first to challenge the regular use of ICS. A pilot study by Papi and colleagues evaluated the efficacy of the symptom-driven use of beclomethasone dipropionate plus albuterol in a single inhaler versus maintenance with inhaled beclomethasone and as-needed albuterol. In this six-month, double-blind, double-dummy, randomized, parallel-group trial, 455 patients with mild asthma were randomized to one of four treatment groups: an as-needed combination therapy of placebo bid plus 250 μg of beclomethasone and 100 μg of albuterol in a single inhaler; an as-needed albuterol combination therapy consisting of placebo bid plus 100 μg of albuterol; regular beclomethasone therapy, comprising beclomethasone 250 μg bid and 100 μg albuterol as needed); and regular combination therapy with beclomethasone 250 μg and albuterol 100 μg in a single inhaler bid plus albuterol 100 μg as needed.
The rescue use of beclomethasone/albuterol in a single inhaler was as efficacious as the regular use of inhaled beclomethasone (250 μg bid ) and it was associated with a lower 6-month cumulative dose of the ICS [ 32 ].
The time to first exacerbation differed significantly among groups ( p = 0.003), with the shortest in the as-needed albuterol and placebo group (Fig. 5 ). Figure 5 also shows equivalence between the as-needed combination therapy and the regular beclomethasone therapy. However, these results were not conclusive since the study was not powered to evaluate the effect of the treatment on exacerbations. In conclusion, as suggested by the study findings, mild asthma patients may require the use of an as-needed ICS and an inhaled bronchodilator rather than a regular treatment with ICS [ 32 ].
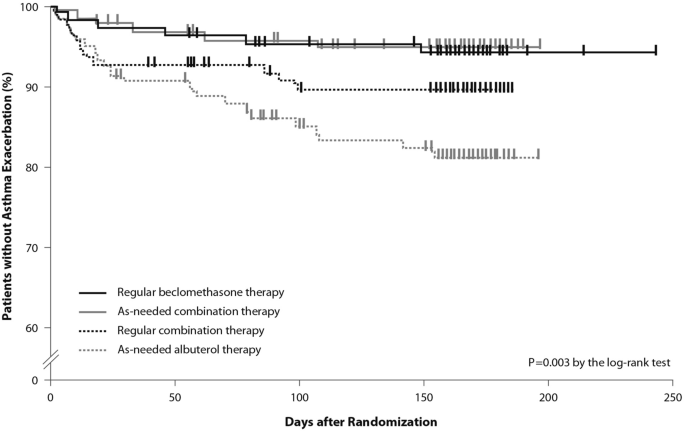
(From [ 32 ])
Kaplan Meier analysis of the time to first exacerbation (modified intention-to-treat population). First asthma exacerbations are shown as thick marks. As-needed albuterol therapy = placebo bid plus 100 μg of albuterol as needed; regular combination therapy = 250 μg of beclomethasone and 100 μg of albuterol in a single inhaler bid plus 100 μg of albuterol as needed; regular beclomethasone therapy = 250 μg of beclomethasone bid and 100 μg of albuterol as needed; as-needed combination therapy = placebo bid plus 250 μg of beclomethasone and 100 μg of albuterol in a single inhaler as needed
Moving forward: a new approach to the management of asthma patients
Nearly a decade after the publication of the BEST study in 2007, the use of this alternative therapeutic strategy was addressed in the SYGMA 1 and SYGMA 2 trials. These double-blind, randomized, parallel-group, 52-week phase III trials evaluated the efficacy of as-needed use of combination formoterol (LABA) and the ICS budesonide as an anti-inflammatory reliever in patients requiring GINA Step 2 treatment, with the current reliever therapy (e.g. as-needed SABA) or with low-dose maintenance ICS (inhaled budesonide bid ) plus as-needed SABA, administered as regular controller therapy [ 33 , 34 ].
The SYGMA 1 trial, which enrolled 3849 patients, aimed to demonstrate the superiority of the as-needed use of the combination budesonide/formoterol over as-needed terbutaline, as measured by the electronically-recorded proportion of weeks with well-controlled asthma [ 34 ]. The more pragmatic SYGMA 2 trial enrolled 4215 patients with the aim to demonstrate that the budesonide/formoterol combination is non-inferior to budesonide plus as-needed terbutaline in reducing the relative rate of annual severe asthma exacerbations [ 33 ]. Both trials met their primary efficacy outcomes. In particular, as-needed budesonide/formoterol was superior to as-needed SABA in controlling asthma symptoms (34.4% versus 31.1%) and preventing exacerbations, achieving a 64% reduction in exacerbations. In both trials, budesonide/formoterol as-needed was similar to budesonide maintenance bid at preventing severe exacerbations, with a substantial reduction of the inhaled steroid load over the study period (83% in the SYGMA 1 trial and 75% in the SYGMA 2 trial). The time to first exacerbation did not differ significantly between the two regimens; however, budesonide/formoterol was superior to SABA in prolonging the time to first severe exacerbation [ 33 , 34 ].
The double-blind, placebo-controlled design of the SYGMA trials does not fully address the advantages of anti-inflammatory reliever strategy in patients who often rely on SABAs for symptom relief, so to what extent the study findings could apply to real-life practice settings was unclear.
These limitations were overcome by the results of the Novel START study, an open-label, randomized, parallel-group, controlled trial designed to reflect real-world practice, which demonstrated the effectiveness in mild asthma of budesonide/formoterol as an anti-inflammatory reliever therapy [ 35 ].
In real-world practice, mild asthma patients are treated with an as-needed SABA reliever or with daily low-dose ICS maintenance therapy plus a SABA reliever. In the Novel START study, 668 patients with mild asthma were randomized to receive either as-needed albuterol 100 µg, two inhalations (SABA reliever as a continuation of the Step 1 treatment according to the 2017 GINA guidelines), budesonide 200 µg (ICS maintenance treatment) plus as-needed albuterol (Step 2 therapy of the GINA 2017 guidelines), or 200 µg/6 µg budesonide/formoterol as anti-inflammatory reliever therapy taken as-needed for a 52-week study period.
In this study, the rate of asthma exacerbations for budesonide/formoterol was lower compared with albuterol (51%) and similar to the twice-daily maintenance budesonide plus albuterol, despite a 52% reduction in the mean steroid dose with the single combination inhaler treatment [ 35 ]. In addition, severe exacerbation rate was lower with budesonide/formoterol as compared with as-needed albuterol and regular twice-daily budesonide. These data support the findings of the SYGMA 1 and 2 trials, highlighting the need for a critical re-examination of current clinical practice. Along with the results of the SYGMA trials, they provide convincing evidence of the advantages of the anti-inflammatory reliever strategy, particularly in real-life settings.
The SYGMA 1, SYGMA 2 and the novel START studies complete the picture of the treatment strategies for asthma at any degree of severity, including mild asthma. A growing body of evidence shows that an anti-inflammatory reliever strategy, when compared with all other strategies with SABA reliever, consistently reduces the rate of exacerbations across all levels of asthma severity (Fig. 6 ) [ 28 , 29 , 34 , 36 , 37 , 38 , 39 ].
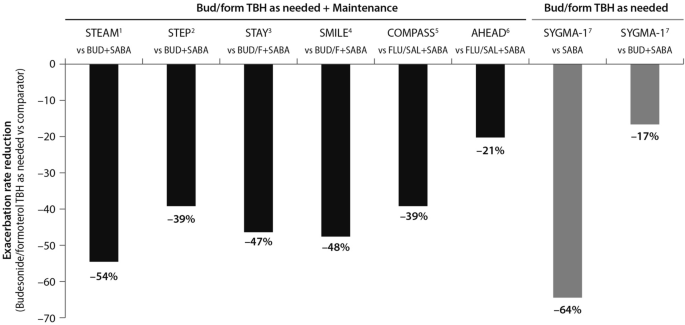
(Data source: [ 39 ])
Risk reduction of severe asthma attack of anti-inflammatory reliever versus SABA across all levels of asthma severity. Bud = budesonide; form = formoterol; TBH = turbohaler. Data from: 1: [ 36 ]; 2: [ 37 ]; 3: [ 38 ]; 4: [ 28 ]; 5: [ 29 ]; 6: [ 30 ]; 7: [ 34 ]
This evidence set the ground (Fig. 7 ) for the release of the 2019 GINA strategy updates. The document provides a consistent approach towards the management of the disease and aims to avoid the overreliance and overuse of SABAs, even in the early course of the disease. The 2019 GINA has introduced key changes in the treatment of mild asthma: for safety reasons, asthmatic adults and adolescents should receive ICS-containing controller treatment instead of the SABA-only treatment, which is no longer recommended.
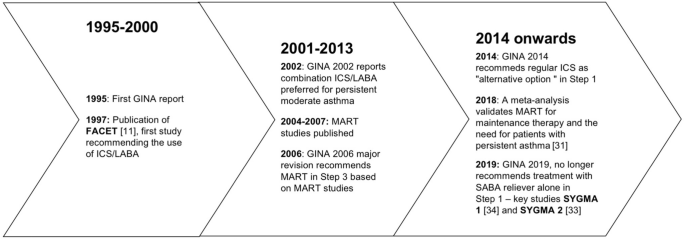
Timeline of key randomized controlled trials and meta-analyses providing the supporting evidence base leading to the Global Initiative for Asthma (GINA) 2019 guidelines. GINA global initiative for asthma, MART maintenance and reliever therapy, SMART single inhaler maintenance and reliever therapy
In Step 1 of the stepwise approach to adjusting asthma treatment, the preferred controller option for patients with fewer than two symptoms/month and no exacerbation risk factors is low-dose ICS/formoterol as needed. This strategy is indirectly supported by the results of the SYGMA 1 study which evaluated the efficacy and safety of budesonide/formoterol as needed, compared with as-needed terbutaline and budesonide bid plus as-needed terbutaline (see above). In patients with mild asthma, the use of an ICS/LABA (budesonide/formoterol) combination as needed provided superior symptom control to as-needed SABA, resulting in a 64% lower rate of exacerbations (p = 0.07) with a lower steroid dose (17% of the budesonide maintenance dose) [ 34 ]. The changes extend to the other controller options as well. In the 2017 GINA guidelines, the preferred treatment was as-needed SABA with the option to consider adding a regular low-dose ICS to the reliever. In order to overcome the poor adherence with the ICS regimen, and with the aim to reduce the risk of severe exacerbations, the 2019 GINA document recommends taking low-dose ICS whenever SABA is taken, with the daily ICS option no longer listed.
Previous studies including the TREXA study in children and adolescents [ 40 ], the BASALT study [ 41 ] and research conducted by the BEST study group [ 32 ] have already added to the evidence that a low-dose ICS with a bronchodilator is an effective strategy for symptom control in patients with mild asthma. A recently published study in African-American children with mild asthma found that the use of as-needed ICS with SABA provides similar asthma control, exacerbation rates and lung function measures at 1 year, compared with daily ICS controller therapy [ 42 ], adding support to TREXA findings that in children with well controlled, mild asthma, ICS used as rescue medication with SABA may be an efficacious step-down strategy [ 40 ].
In Step 2 of the stepwise approach, there are now two preferred controller options: (a) a daily low-dose ICS plus an as-needed SABA; and (b) as-needed low-dose ICS/formoterol. Recommendation (a) is supported by a large body of evidence from randomized controlled trials and observations showing a substantial reduction of exacerbation, hospitalization, and death with regular low-dose ICS [ 7 , 8 , 9 , 24 , 43 ], whereas recommendation (b) stems from evidence on the reduction or non-inferiority for severe exacerbations when as-needed low-dose ICS/formoterol is compared with regular ICS [ 33 , 34 ].
The new GINA document also suggests low-dose ICS is taken whenever SABA is taken, either as separate inhalers or in combination. This recommendation is supported by studies showing reduced exacerbation rates compared with taking a SABA only [ 32 , 40 ], or similar rates compared with regular ICS [ 32 , 40 , 41 ]. Low-dose theophylline, suggested as an alternative controller in the 2017 GINA guidelines, is no longer recommended.
Airway inflammation is present in the majority of patients with asthma, and although patients with mild asthma may have only infrequent symptoms, they face ongoing chronic inflammation of the lower airways and risk acute exacerbations. The GINA 2019 strategy recognizes the importance of reducing the risk of asthma exacerbations, even in patients with mild asthma (Steps 1 and 2) [ 4 ]. In this regard, the new recommendations note that SABA alone for symptomatic treatment is non-protective against severe exacerbation and may actually increase exacerbation risk if used regularly or frequently [ 4 ].
The reluctance by patients to regularly use an ICS controller means they may instead try and manage their asthma symptoms by increasing their SABA reliever use. This can result in SABA overuse and increased prescribing, and increased risk of exacerbations.
As part of the global SABINA (SABA use IN Asthma) observational study programme, a UK study examined primary care records to describe the pattern of SABA and ICS use over a 10-year period in 373,256 patients with mild asthma [ 44 ]. Results showed that year-to-year SABA prescribing was more variable than that of ICS indicating that, in response to fluctuations in asthma symptom control, SABA use was increased in preference to ICS use. Furthermore, more than 33% of patients were prescribed SABA inhalers at a level equivalent to around ≥ 3 puffs per week which, according to GINA, suggests inadequate asthma control.
The problem of SABA overuse is further highlighted by two studies [ 45 , 46 ], also as part of the SABINA programme. These analysed data from 365,324 patients in a Swedish cohort prescribed two medications for obstructive lung disease in any 12-month period (HERA).
The first study identified SABA overuse (defined as ≥ 3 SABA canisters a year) in 30% of patients, irrespective of their ICS use; 21% of patients were collecting 3–5 canisters annually, 7% were collecting 6–10, and 2% more than 11 [ 45 ]. Those patients who were overusing SABA had significantly more asthma exacerbations relative to those using < 3 canisters (20.0 versus 12.5 per 100 patient years; relative risk 1.60, 95% CI 1.57–1.63, p < 0.001). Moreover, patients overusing SABA and whose asthma was more severe (GINA Steps 3 and 4) had greater exacerbation risk compared with overusing patients whose asthma was milder (GINA Steps 1 and 2).
The second study found those patients using three or more SABA reliever canisters a year had an increased all-cause mortality risk relative to patients using fewer SABA canisters: hazard ratios after adjustment were 1.26 (95% CI 1.14–1.39) for 3–5 canisters annually, 1.67 (1.49–1.87) for 6–10 canisters, and 2.35 (2.02–2.72) for > 11 canisters, relative to patients collecting < 3 canisters annually [ 46 ].
The recently published PRACTICAL study lends further support to as-needed low-dose ICS/formoterol as an alternative option to daily low-dose ICS plus as-needed SABA, outlined in Step 2 of the guidelines [ 47 ]. In their one-year, open-label, multicentre, randomized, superiority trial in 890 patients with mild to moderate asthma, Hardy and colleagues found that the rate of severe exacerbations per patient per year (the primary outcome) was lower in patients who received as-needed budesonide/formoterol than in patients who received controller budesonide plus as-needed terbutaline (relative rate 0.69, 95% CI 0.48–1.00; p < 0.05). Indeed, they suggest that of these two treatment options, as-needed low-dose ICS/formoterol may be preferred over controller low-dose ICS plus as-needed SABA for the prevention of severe exacerbations in this patient population.
Step 3 recommendations have been left unchanged from 2017, whereas Step 4 treatment has changed from recommending medium/high-dose ICS/LABA [ 3 ] to medium-dose ICS/LABA; the high-dose recommendation has been escalated to Step 5. Patients who have asthma that remains uncontrolled after Step 4 treatment should be referred for phenotypic assessment with or without add-on therapy.
To summarise, the use of ICS medications is of paramount importance for optimal asthma control. The onset and increase of symptoms are indicative of a worsening inflammation leading to severe exacerbations, the risk of which is reduced by a maintenance plus as-needed ICS/LABA combination therapy. The inhaled ICS/bronchodilator combination is as effective as the regular use of inhaled steroids.
The efficacy of anti-inflammatory reliever therapy (budesonide/formoterol) versus current standard-of-care therapies in mild asthma (e.g. reliever therapy with a SABA as needed and regular maintenance controller therapy plus a SABA as-needed) has been evaluated in two randomized, phase III trials which confirmed that, with respect to as-needed SABA, the anti-inflammatory reliever as needed is superior in controlling asthma and reduces exacerbation rates, exposing the patients to a substantially lower glucocorticoid dose.
Conclusions
A growing body of evidence shows that anti-inflammatory reliever strategy is more effective than other strategies with SABA reliever in controlling asthma and reducing exacerbations across all levels of asthma severity. A budesonide/formoterol therapy exposes asthma patients to a substantially lower glucocorticoid dose while cutting the need for adherence to scheduled therapy.
Availability of data and materials
Not applicable.
Abbreviations
Global Initiative for Asthma
Inhaled corticosteroids
Long-acting beta-agonists
Oral corticosteroids
Short-acting beta-agonists
Single inhaler maintenance and reliever treatment
World Health Organization. Asthma. 2017. https://www.who.int/news-room/fact-sheets/detail/asthma . Accessed 9 April 2019.
Papi A, Brightling C, Pedersen SE, Reddel HK. Asthma. Lancet. 2018;391(10122):783–800.
Article PubMed Google Scholar
Global Initiative for Asthma. Global strategy for asthma management and prevention, 2017. http://www.ginasthma.org . Accessed 1 June 2019.
Global Initiative for Asthma. Pocket guide for asthma management and prevention. 2019, pp. 1–32. www.ginasthma.org . Accessed 1 June 2019.
Rabe KF, Vermeire PA, Soriano JB, Maier WC. Clinical management of asthma in 1999: the Asthma Insights and Reality in Europe (AIRE) study. Eur Respir J. 2000;16(5):802–7.
Article CAS PubMed Google Scholar
Price D, Fletcher M, Molen V. Asthma control and management in 8,000 European patients: the REcognise Asthma and LInk to Symptoms and Experience (REALISE) survey. NPJ Prim Care Respir Med. 2014;24:14009.
Article PubMed Central PubMed Google Scholar
Suissa S, Ernst P, Kezouh A. Regular use of inhaled corticosteroids and the long term prevention of hospitalisation for asthma. Thorax. 2002;57(10):880–4.
Article CAS PubMed Central PubMed Google Scholar
Suissa S, Ernst P, Benayoun S, Baltzan M, Cai B. Low-dose inhaled corticosteroids and the prevention of death from asthma. New Engl J Med. 2000;343(5):332–6.
Pauwels RA, Pedersen S, Busse WW, Tan WC, Chen YZ, Ohlsson SV, et al. Early intervention with budesonide in mild persistent asthma: a randomised, double-blind trial. Lancet. 2003;361(9363):1071–6.
Healthcare Improvement Scotland and British Thoracic Society. SIGN 153: British guideline on the management of asthma. 2016. https://www.sign.ac.uk/sign-153-british-guideline-on-the-management-of-asthma.html .
Pauwels RA, Löfdahl CG, Postma DS, Tattersfield AE, O’Byrne P, Barnes PJ, Ullman A. Effect of inhaled formoterol and budesonide on exacerbations of asthma. N Engl J Med. 1997;337(20):1405–11.
O’Byrne PM, Naya IP, Kallen A, Postma DS, Barnes PJ. Increasing doses of inhaled corticosteroids compared to adding long-acting inhaled β2-agonists in achieving asthma control. Chest. 2008;134(6):1192–9.
Johnston SL, Pattemore PK, Sanderson G, Smith S, Lampe F, Josephs L, et al. Community study of role of viral infections in exacerbations of asthma in 9–11 year old children. BMJ. 1995;310(6989):1225–9.
Corne JM, Marshall C, Smith S, Schreiber J, Sanderson G, Holgate ST, Johnston SL. Frequency, severity, and duration of rhinovirus infections in asthmatic and non-asthmatic individuals: a longitudinal cohort study. Lancet. 2002;359(9309):831–4.
Papi A, Contoli M, Adcock IM, Bellettato C, Padovani A, Casolari P, et al. Rhinovirus infection causes steroid resistance in airway epithelium through nuclear factor κb and c-Jun N-terminal kinase activation. J Allergy Clin Immunol. 2013;132(5):1075–85.
Romagnoli M, Vachier I, Tarodo de la Fuente P, Meziane H, Chavis C, Bousquet J, et al. Eosinophilic inflammation in sputum of poorly controlled asthmatics. Eur Respir J. 2002;20(6):1370–7.
Partridge MR, van der Molen T, Myrseth SE, Busse WW. Attitudes and actions of asthma patients on regular maintenance therapy: the INSPIRE study. BMC Pulm Med. 2006;6:13.
Papi A, Caramori G, Adcock IM, Barnes PJ. Rescue treatment in asthma. More than as-needed bronchodilation. Chest. 2009;135(6):1628–33.
PubMed Google Scholar
Tattersfield AE, Postma DS, Barnes PJ, Svensson K, Bauer CA, Byrne PM, et al. Exacerbations of asthma: a descriptive study of 425 severe exacerbations The FACET International Study Group. Am J Respir Crit Care Med. 1999;160(2):594–9.
O’Byrne PM, Jenkins C, Bateman ED. The paradoxes of asthma management: time for a new approach? Eur Respir J. 2017. https://doi.org/10.1183/13993003.01103-2017 .
Royal College of Physicians. Why asthma still kills: the National Review of Asthma Deaths (NRAD). Confidential Enquiry report. London: RCP; 2014.
Google Scholar
Suissa S, Ernst P, Boivin JF, Horwitz RI, Habbick B, Cockroft D, et al. A cohort analysis of excess mortality in asthma and the use of inhaled β-agonists. Am J Respir Crit Care Med. 1994;149(3 Pt 1):604–10.
Global Initiative for Asthma. Global strategy for asthma management and prevention, 2014. https://ginasthma.org/wp-content/uploads/2019/01/2014-GINA.pdf .
Reddel HK, Busse WW, Pedersen S, Tan WC, Chen YZ, Jorup C, et al. Should recommendations about starting inhaled corticosteroid treatment for mild asthma be based on symptom frequency: a post hoc efficacy analysis of the START study. Lancet. 2017;389(10065):157–66.
Papi A, Fabbri LM. Management of patients with early mild asthma and infrequent symptoms. Lancet. 2017;389(10065):129–30.
Global Initiative for Asthma. Global strategy for asthma management and prevention, 2016. https://ginasthma.org/wp-content/uploads/2016/04/GINA-Appendix-2016-final.pdf .
Krishnan JA, Riekert KA, McCoy JV, Stewart DY, Schmidt S, Chanmugam A, et al. Corticosteroid use after hospital discharge among high-risk adults with asthma. Am J Respir Crit Care Med. 2004;170(12):1281–5.
Rabe KF, Atienza T, Magyar P, Larsson P, Jorup C, Lalloo UG. Effect of budesonide in combination with formoterol for reliever therapy in asthma exacerbations: a randomised controlled, double-blind study. Lancet. 2006;368(9537):744–53.
Kuna P, Peters MJ, Manjra AI, Jorup C, Naya IP, Martínez-Jimenez NE, Buhl R. Effect of budesonide/formoterol maintenance and reliever therapy on asthma exacerbations. Int J Clin Pract. 2007;61(5):725–36.
Bousquet J, Boulet LP, Peters MJ, Magnussen H, Quiralte J, Martinez-Aguilar NE, Carlsheimer A. Budesonide/formoterol for maintenance and relief in uncontrolled asthma vs. high-dose salmeterol/fluticasone. Respir Med. 2007;101(12):2437–46.
Sobieraj DM, Weeda ER, Nguyen E, Coleman CI, White CM, Lazarus SC, et al. Association of inhaled corticosteroids and long-acting β-agonists as controller and quick relief therapy with exacerbations and symptom control in persistent asthma a systematic review and meta-analysis. JAMA. 2018;319(14):1485–96.
Papi A, Canonica GW, Maestrelli P, Paggiaro P, Olivieri D, Pozzi E, BEST Study Group, et al. Rescue use of beclomethasone and albuterol in a single inhaler for mild asthma. New Engl J Med. 2007;356(20):2040–52.
Bateman ED, Reddel HK, O’Byrne PM, Barnes PJ, Zhong N, Keen C, et al. As-needed budesonide–formoterol versus maintenance budesonide in mild asthma. New Engl J Med. 2018;378(20):1877–87.
O’Byrne PM, FitzGerald JM, Bateman ED, Barnes PJ, Zhong N, Keen C, et al. Inhaled combined budesonide-formoterol as needed in mild asthma. New Engl J Med. 2018;378(20):1865–76.
Beasley R, Holliday M, Reddel HK, Braithwaite I, Ebmeier S, Hancox RJ, Novel START Study Team, et al. Controlled trial of budesonide-formoterol as needed for mild asthma. New Engl J Med. 2019;380(21):2020–30.
Rabe K, Pizzichini E, Ställberg B, Romero S, Balanzat AM, Atienza T, et al. Budesonide/formoterol in a single inhaler for maintenance and relief in mild-to-moderate asthma: a randomized, double-blind trial. Chest. 2006;129(2):246–56.
Scicchitano R, Aalbers R, Ukena D, Manjra A, Fouquert L, Centanni S, et al. Efficacy and safety of budesonide/formoterol single inhaler therapy versus a higher dose of budesonide in moderate to severe asthma. Curr Med Res Opin. 2004;20(9):1403–18.
O’Byrne PM, Bisgaard H, Godard PP, Pistolesi M, Palmqvist M, Zhu Y, et al. Budesonide/formoterol combination therapy as both maintenance and reliever medication in asthma. Am J Respir Crit Care Med. 2005;171(2):129–36.
Pavord ID, Beasley R, Agusti A, Anderson GP, Bel E, Brusselle G, et al. After asthma: redefining airways diseases. Lancet. 2017;391(10118):350–400.
Martinez FD, Chinchilli VM, Morgan WJ, Boehmer SJ, Lemanske RF Jr, Mauger DT, et al. Use of beclomethasone dipropionate as rescue treatment for children with mild persistent asthma (TREXA): a randomised, double-blind, placebo-controlled trial. Lancet. 2011;377(9766):650–7.
Calhoun WJ, Ameredes BT, King TS, Icitovic N, Bleecker ER, Castro M, et al. Comparison of physician-, biomarker-, and symptom-based strategies for adjustment of inhaled corticosteroid therapy in adults with asthma: the BASALT randomized controlled trial. JAMA. 2012;308(10):987–97.
Sumino K, Bacharier LB, Taylor J, et al. A pragmatic trial of symptom-based inhaled corticosteroid use in African-American children with mild asthma. J Allergy Clin Immunol Pract. 2020;8(176–85):e2.
Byrne PM, Barnes PJ, Rodriguez-Roisin R, Runnerstrom E, Sandstrom T, Svensson K, Tattersfield A. Low dose inhaled budesonide and formoterol in mild persistent asthma: the OPTIMA randomized trial. Am J Respir Crit Care Med. 2001;164(8):1392–7.
Article Google Scholar
Bloom C, Quint J, Cabrera C. SABA and ICS prescriptions among mild asthma patients in UK primary care. Poster presented at the European Respiratory Society International Congress; 2019 Sep 28–Oct 2; Madrid, Spain.
Janson C, Nwaru B, Hasvold P, Wicklund F, Telg G, Ekstrom M. Use of short-acting beta-2 agonists (SABA) and exacerbations in a nationwide Swedish asthma cohort (HERA). Poster presented at the European Respiratory Society International Congress; 2019 Sep 28–Oct 2; Madrid, Spain.
Janson C, Nwaru B, Hasvold P, Wicklund F, Telg G, Ekstrom M. SABA overuse and risk of mortality in a nationwide Swedish asthma cohort (HERA). Late Breaker abstract at the European Respiratory Society International Congress; 2019 Sep 28–Oct 2; Madrid, Spain.
Hardy J, Baggott C, Fingleton J, Reddel HK, Hancox RJ, Harwood M, et al. Budesonide-formoterol reliever therapy versus maintenance budesonide plus terbutaline reliever therapy in adults with mild to moderate asthma (PRACTICAL): a 52-week, open-label, multicentre, superiority, randomised controlled trial. Lancet. 2019;394(10202):919–28.
Download references
Acknowledgements
The Authors thank Maurizio Tarzia and Gayle Robins, independent medical writers who provided editorial assistance on behalf of Springer Healthcare Communications. The editorial assistance was funded by AstraZeneca.
No funding was received for this study. The editorial assistance was funded by AstraZeneca.
Author information
Authors and affiliations.
Section of Cardiorespiratory and Internal Medicine, Department of Morphology, Surgery and Experimental Medicine, University of Ferrara, Ferrara, Italy
Alberto Papi & Luca Morandi
Internal Medicine Department, Respiratory Unit and Adult Cystic Fibrosis Center, Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico, Milan, Italy
Francesco Blasi
Department of Pathophysiology and Transplantation, Università degli Studi di Milano, Milan, Italy
Personalized Medicine Asthma & Allergy Clinic, Humanitas University & Istituto Clinico Humanitas, Milan, Italy
Giorgio Walter Canonica
Università Cattolica del Sacro Cuore, Fondazione Policlinico A. Gemelli IRCCS, Rome, Italy
Luca Richeldi
Respiratory Section, Department of Medicine, University of Verona, Verona, Italy
Andrea Rossi
Respiratory Unit, Emergency Department, University Hospital S. Anna, Via Aldo Moro 8, 44124, Ferrara, Italy
You can also search for this author in PubMed Google Scholar
Contributions
AP, FB, GWC, LM, LR and AR contributed to writing. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Alberto Papi .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
AP reports grants, personal fees, non-financial support and payment for advisory board membership, consultancy, payment for lectures, grants for research, and travel expenses reimbursement from Chiesi, AstraZeneca, GlaxoSmithKline, Boehringer Ingelheim, Mundipharma and Teva, and personal fees and non-financial support from Menarini, Novartis, Zambon and Sanofi.
FB reports having received in the last three years research grants as well as lecture or advisory board fees from: Alk-Abelló, AstraZeneca, Boehringer Ingelheim, Chiesi, Guidotti, Glaxo Smith Kline, Grifols, Menarini, Novartis, Sanofi, Valeas, Zambon.
GWC reports having received in the last 3 years research grants as well as lecture or advisory board fees from: A. Menarini, Alk-Abelló, AstraZeneca-Medimmune, Boehringer Ingelheim, Chiesi Farmaceutici, Genentech, Guidotti-Malesci, Glaxo Smith Kline, Hal Allergy, Merck Sharp & Dohme, Mundipharma, Novartis, Orion, Sanofi-Aventis, Sanofi Genzyme/Regeneron, Stallergenes-Greers, UCB Pharma, Uriach Pharma, Valeas.
LR Receipt of grants/research supports: Roche, Boehringer Ingelheim.
Receipt of honoraria or consultation fees: Boehringer Ingelheim, Roche, Biogen, FibroGen,
Sanofi-Aventis, Anthera, Promedior, ImmuneWorks, Asahi-Kasei, Bayer, Celgene, RespiVant,
Nitto, Bristol Myers Squibb, Prometic, Pliant Therapeutics, Toray, Global Blood Therapeutics,
Zambon, Veracyte, Acceleron, CSL Behring.
LM and AR reports no conflicts of interest in the last 3 years.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Papi, A., Blasi, F., Canonica, G.W. et al. Treatment strategies for asthma: reshaping the concept of asthma management. Allergy Asthma Clin Immunol 16 , 75 (2020). https://doi.org/10.1186/s13223-020-00472-8
Download citation
Received : 18 March 2020
Accepted : 05 August 2020
Published : 15 August 2020
DOI : https://doi.org/10.1186/s13223-020-00472-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Anti-inflammatory treatment
- Disease control
- Patient outcomes
Allergy, Asthma & Clinical Immunology
ISSN: 1710-1492
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Home Essay Examples Health Asthma
Asthma And Its Treatment
- Category Health
- Subcategory Disease
- Topic Asthma

Introduction
Asthma is a complex heterogeneous disease, characterised by chronic airway inflammation and recurrent, episodic respiratory exacerbations, namely wheezing, breathlessness, chest tightness and coughing, particularly at night (Kaufman, 2011). Danvers et al (2019) describe asthma as being the most prevalent chronic condition affecting children within the United Kingdom (UK), with 374 child deaths (14 years and under) being reported between 2001 and 2016 (Asthma UK, 2020a). Remarkably, Asthma UK (2020b) claims that two-thirds of the aforementioned fatalities could be prevented if better asthma control measures were implemented (Leyshon, 2011). Kai, who is at the heart of this assignment, is a fourteen-year-old male, diagnosed with asthma, attention-deficit/hyperactivity disorder (AD/HD) and a mild learning disability (MLD). Due to the severe risks associated with asthma, Kai’s ‘nocturnal cough’ has been selected as the principal focus of the care plan. Over 12 weeks, the implementation of four evidence-based interventions aim to reduce the overall frequency (1-2 episodes a week, to 1-2 a month) and severity of Kia’s nocturnal cough. To do that, the following interventions will be employed; the creation of a therapeutic relationship, medication administration technique, peak expiratory flow (PEF)/medication monitoring and smoking cessation referral. Throughout, recognition of Kai’s behavioural and learning needs will be discussed, by considering how they may impact his adherence to the plan, and his life at both home and school.
Development of a therapeutic relationship
Crotty and Doody (2015), recommend that the formation of a therapeutic, nurse-patient relationship, should be considered a prerequisite when striving for high quality of care outcomes. This is congruent with Rogers (2004), who states that the adoption of a person-centred approach often maximises therapeutic value and adherence. Significantly, the distractible, disorganised and impulsive nature of Kai’s AD/HD, means that forming relationships is often difficult (NICE, 2018a). Therefore, in an attempt to facilitate meaningful conversations with Kai, it is paramount to create a safe environment, through congruence and trust (Rogers, 2004). Engaging Kia in this manner, allows healthcare practitioners to assume the role of active listener and attentive partner, thus unambiguously comprehending Kai’s individualist needs (Crotty and Doody, 2015). Prioritising Kai’s preferences and concerns, ensures a truly personalised care plan. Challenges arise when deciding which communication strategy to employ. Greenhalgh and Heath (2010), advise caution, suggesting that designating a specific model for building client relationships is unpragmatic. There is no ‘one-fits-all approach’, which reiterates the importance of sound clinical judgement and its subsequent application in practice (NMC, 2019). Evidently, building a therapeutic relationship can be time-consuming. Moreover, Stothard (2017) revealed that asthma patients with MLD, need longer consultations than those without MLD. When patients were afforded more time, reductions in the use of emergency healthcare resources were reported. Crucially, through empathetic guidance, Kia and his family may be better equipped to recognise how his conditions interact and influence his interpersonal relationships. Specifically, his neurodevelopment, and mental health, now and in the future (Wenderlich et al, 2019).
Our writers can write you a new plagiarism-free essay on any topic
Inhaler Devices Technique/Suitability
A classical feature of asthma is inflammation and bronchoconstriction of the respiratory airways, due to an overzealous immuno-response (Barber and Robertson, 2015); often triggered by allergen exposure or other mechanisms, resulting in airflow obstruction (NICE, 2018b). In accordance with NICE guidelines (2017), Kai has been prescribed short-acting-beta-2-agonist (SABA) medication, namely Salbutamol, in an attempt to reverse these effects. Despite being the mainstay of treatment, Finkelstein et al (2009), asserts that individual responses to SABA’s are variable and difficult to predict. Significantly, Khan et al (2017), found that the proliferation of nocturnal symptoms could be associated with fluctuations in beta-2-adrenergic receptor sensitivity at night (Khan et al, 2017). Therefore, to address the issue of reducing Kai’s nocturnal cough, every effort needs to be made to ensure optimised medication delivery, to the required site of action (Usmani, 2019). The Thoracic Society and Scottish Intercollegiate Guidelines Network (BTS/SIGN) (2011), recommend that inhaled asthma medication should not be prescribed without inhaler patients being competent in their use. Thus, it is paramount to ensure training is provided to Kai, with his technique being regularly assessed, to reaffirm its effective use. Additionally, Rollnick et al (2005), outlines the importance of encouraging patients to explore their views and goals regarding treatment. Crucially, determining what an acceptable device looks like to Kai, and whether it suits his needs is imperative. Moreover, adopting a patient-centred approach avoids practitioners making assumptions about Kai’s capacity to use a particular device (Leyshon, 2011). Striking a balance between accommodating Kai’s choices and the impact his AD/HD and MLD might have on device operation is key. Responding to Kai’s individual preferences, in accordance with section 2 of the NMC code (2018), will likely improve his medication adherence and compliance over the long term (Fletcher et al, 2005; Kaplan and Price, 2020). Likewise, to promote positive behaviour change, Kai’s care plan must be underpinned by realistic timeframes. Analysis by Lully et al (2009), discovered that on average, habit formation took 66 days (but ranged from 18-254 days). Therefore, the proposed 12-week plan falls within these recommended parameters.
Peak Expiratory Flow (PEF) and Medication Monitoring
Kai’s nocturnal cough is indicative of poorly controlled asthma (Leyshon, 2011). Moreover, to limit the severity and frequency of future ‘attacks’, it is vital that we make every attempt to explore potential underlying causes. Conceivably, Kai may experience adherence challenges, or may not be aware of his triggers. According to NICE (2009), non-adherence falls into two distinct categories; intentional (intrinsic motives/beliefs) and unintentional (barriers outside of the patient’s control). Therefore, to develop a wholly unique personal asthma action plan (PAAP) (Newell et al, 2015), objective data must be collated (Holmes, 2017). NICE (2009), propose the use of a medication and symptom diary, in conjunction with PEF charting. Despite its limitations, PEF is a simple, economical test that can be used to monitor lung function in asthma patients (Booker, 2007; Asthma UK, 2016a). However, due to asthmatic variability, it is not possible to gauge a ‘normal’ PEF score with a single assessment. Booker (2007) recommends that serial PEF readings be recorded morning and evening, for a period of no less than 2-3 weeks. Scores can then be used to assess diurnal and nocturnal variability (BTS/SIGN, 2019), providing objective data that underpins Kai’s PAAP. Significantly, the detail at which Kai is required to monitor his condition may present problems. Potential solutions exist through smartphone asthma applications (Huckvale et al, 2015). Interestingly, in 2019, 96% of 16-24 years old in the UK owned a smartphone (O’Dea, 2019). Asthma UK (2016b) view this kind of connectedness in data collection, as an opportunity that could greatly enhance clinical care outcomes, whilst supporting individuals in self-management. Notwithstanding Kai being the focus of the care plan, consideration must be given as to how Kai’s conditions impact life at home and school. Where appropriate, families should be engaged in his treatment, ensuring a holistic support network for all involved (UKAP, 2019).
Smoking Cessation Referral
Smoking is a leading cause of preventable death in the UK and has huge economic costs to the National Health Service (NHS) (Bauld et al, 2009). Worryingly, Boulet et al (2006) report that smoking among asthma patients is relatively common, with a prevalence similar to that of the general population. Moreover, it is argued that smoking could be viewed as the most important modifiable risk factor associated with improving asthma control (Asthma UK, 2018), due primarily to its toxic and proinflammatory effects (Boulet et al, 2006). Similarly, smoking has been found to interfere with the pharmacotherapeutic responses to corticosteroids, thus increasing symptom severity (Spears et al, 2013). Most adolescents would like to stop smoking (Asthma UK, 2018), but with relapse rates being high (Harvey and Chadi, 2016), employing a strategy that engages Kai when considering his long-term outcomes is crucial. Smoking Cessation Services (SCS) are an intervention advocated by NICE (2018) as an effective framework for assisting adolescents, like Kai, to stop smoking. Furthermore, being mindful of Kai’s personal needs and preferences may enhance the chances of him agreeing to SCS referral (NCSCT, 2010). Engaging Kai, by asking, “Why do you smoke?” and “Have you thought about giving up?”, open up dialogue, allowing us to understand his reasons for smoking; for example, does he do it to ‘fit in’ with his peers at school? Equally, it is important to consider intrinsic and extrinsic barriers that may impede Kai’s adherence to an SCS programme. For instance, Bauld et al (2009), report that socioeconomic factors are a strong driver in SCS outcomes. Notably, Kai’s mother has failed to attend appointments in the past, citing ‘transport issues’ as a reason. Summarily, having captured Kai’s information, it must then be delivered to SCS. To do this, NHS guidelines recommend the use of the SBAR communication tool (NHS, 2018), which supports the prompt transfer of accurate patient information to relevant departments.
Asthma is a highly complex, chronic disease that is widespread among the UK populous (Asthma, 2020a). With poor symptom control comes an increased risk of severe exacerbations and/or death (Leyshon, 2011). The primary approach employed in improving health outcomes for asthma suffers focuses on symptom control, through the implementation of varying pharmacological (NICE, 2017), and non-pharmacological interventions (NICE, 2018). Consequently, Kai’s care plan has been constructed in a way that utilises various approaches, to reduce the frequency and severity of his nocturnal symptoms over a period of 12-weeks. Firstly, Kai’s care plan considers his unique behavioural and learning needs, through the development of a ‘nurse-patient’ relationship. Despite Kai being the focus of the plan, it recognised that a collaborative effort is essential; hence the inclusion of Kai’s family throughout the planning and treatment process. In accordance with NICE (2017), immediate assessment of Kai’s inhaler technique is deemed critical, in ensuring optimised medication delivery for acute symptom relief. Capturing PEF measures and medication usage data over a 12-week period, allows for the objective assessment of both the efficacy of his prescription drugs and his symptom control. Moreover, the serious detrimental effects smoking has on Kai’s health has not been overlooked. A SCS referral allows for behavioural specialists to fully explore Kai’s reasons for smoking and his readiness to stop.
We have 98 writers available online to start working on your essay just NOW!
Related Topics
Related essays.
By clicking "Send essay" you agree to our Terms of service and Privacy statement . We will occasionally send you account related emails.
By clicking "Receive essay" you agree to our Terms of service and Privacy statement . We will occasionally send you account related emails.
We can edit this one and make it plagiarism-free in no time
We use cookies to give you the best experience possible. By continuing we’ll assume you board with our cookie policy .
Home — Essay Samples — Nursing & Health — Asthma — Asthma: Causes, Pathophysiology, and Treatment
Asthma: Causes, Pathophysiology, and Treatment
- Categories: Asthma
About this sample

Words: 635 |
Published: Apr 2, 2020
Words: 635 | Page: 1 | 4 min read
Table of contents
Introduction, pathophysiology, classification, management and treatment, lifestyle modification, medications, drug used to treat asthma, ipratropium bromide.

Cite this Essay
Let us write you an essay from scratch
- 450+ experts on 30 subjects ready to help
- Custom essay delivered in as few as 3 hours
Get high-quality help

Dr. Karlyna PhD
Verified writer
- Expert in: Nursing & Health

+ 120 experts online
By clicking “Check Writers’ Offers”, you agree to our terms of service and privacy policy . We’ll occasionally send you promo and account related email
No need to pay just yet!
Related Essays
6 pages / 2514 words
7 pages / 3056 words
3 pages / 1223 words
3 pages / 1222 words
Remember! This is just a sample.
You can get your custom paper by one of our expert writers.
121 writers online
Still can’t find what you need?
Browse our vast selection of original essay samples, each expertly formatted and styled
Related Essays on Asthma
Mark Romanek explores the difficult choices that people make when faced with death in his film Never Let Me Go (2010). He explores the raw human emotions of jealousy and forgiveness through the characterisation of Ruth (Keira [...]
Roe vs. Wade was a law established in America on January 22nd, 1973. This court decision allowed women to be able to get abortions in the first trimester. Despite this, the debate on whether abortion should be legal is still [...]
In his Letter to Menoeceus, Epicurus outlines his philosophy of attaining happiness and details the proper attitude that Epicureans should have toward the gods and toward death. In reference to the latter, following his [...]
In 2013, Venka Child aged 16 from Bristol worked with Fixers to create a short video about challenges teen mothers go through. In some part of the video, a teen mother is shown opening a fridge which is almost empty. The teen [...]
Of the dozens of videos you watch every day, how many do you actually remember? The goal of this PSA video is to be one that you would remember. A good PSA is strong, genuine, and powerful enough to leave an impression . To [...]
The Roles of Death and Mortality in “Because I could not stop for Death” Emily Dickinson’s “Because I could not stop for Death” deals with two interrelated yet distinct subjects: death and mortality. The poet presents these [...]
Related Topics
By clicking “Send”, you agree to our Terms of service and Privacy statement . We will occasionally send you account related emails.
Where do you want us to send this sample?
By clicking “Continue”, you agree to our terms of service and privacy policy.
Be careful. This essay is not unique
This essay was donated by a student and is likely to have been used and submitted before
Download this Sample
Free samples may contain mistakes and not unique parts
Sorry, we could not paraphrase this essay. Our professional writers can rewrite it and get you a unique paper.
Please check your inbox.
We can write you a custom essay that will follow your exact instructions and meet the deadlines. Let's fix your grades together!
Get Your Personalized Essay in 3 Hours or Less!
We use cookies to personalyze your web-site experience. By continuing we’ll assume you board with our cookie policy .
- Instructions Followed To The Letter
- Deadlines Met At Every Stage
- Unique And Plagiarism Free
- Skip to content
- skip to navigation
FASENRA approved for treatment of children aged 6 to 11 with severe asthma
Additional indication for pediatric patients with severe eosinophilic asthma
AstraZeneca’s FASENRA ® (benralizumab) is now approved by the US Food and Drug Administration (FDA) for add-on maintenance treatment for patients with severe asthma aged 6 to 11 with an eosinophilic phenotype. 1 FASENRA was first approved in 2017 as an add-on maintenance for the treatment of severe eosinophilic asthma (SEA) in patients aged 12 and older. 1
This additional indication for FASENRA was supported by evidence from TATE, an open-label, multinational, non-randomized, parallel assignment Phase III trial, as well as adequate and well-controlled trials in adult and adolescent populations. 2 In the TATE study, FASENRA met the primary endpoints, demonstrating pharmacokinetics (PK) and pharmacodynamics (PD) in children aged 6 to 11 years old with SEA were consistent with those seen in prior trials. The safety and tolerability of FASENRA in the trial was also consistent with the known profile of the medicine. 2 The recommended dose for FASENRA is 30 mg for patients 6 years and older who weigh 35 kg or more. For patients aged 6 to 11 who weigh less than 35 kg, a new 10 mg dose will be available. 1 FASENRA is administered by subcutaneous injection every 4 weeks for the first 3 doses, and then every 8 weeks.
Lynda Mitchell, MA, CAE, CEO, of the Allergy & Asthma Network, said: “We welcome additional treatment options for children living with severe asthma, a condition that remains complicated to manage, further helping to address the unmet need in this patient population and reducing the burden of disease for the broader asthma community.”
Asthma is the most common chronic childhood disease and can cause serious symptoms such as coughing, wheezing and difficulty breathing. 3 Children with severe asthma and their families face a significant burden, including impaired school performance, substantially higher healthcare resource use and a poorer quality of life. 4 Severe asthma is a debilitating type of asthma that can be complicated and challenging to treat. 4
Liz Bodin, Vice President, US Respiratory & Immunology, AstraZeneca said: “We’re proud that FASENRA has helped more than 100,000 patients in the US to date. Expanding options for children whose quality of life has been drastically impacted by severe eosinophilic asthma with the help of FASENRA is an exciting step in our mission to revolutionize asthma care.”
FASENRA is currently approved as an add-on maintenance treatment for patients aged 6 and older with SEA in the US. 1
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS
Known hypersensitivity to benralizumab or excipients.
WARNINGS AND PRECAUTIONS
Hypersensitivity Reactions
Hypersensitivity reactions (e.g., anaphylaxis, angioedema, urticaria, rash) have occurred after administration of FASENRA. These reactions generally occur within hours of administration, but in some instances have a delayed onset (i.e., days). Discontinue in the event of a hypersensitivity reaction.
Acute Asthma Symptoms or Deteriorating Disease
FASENRA should not be used to treat acute asthma symptoms, acute exacerbations, or acute bronchospasm.
Reduction of Corticosteroid Dosage
Do not discontinue systemic or inhaled corticosteroids abruptly upon initiation of therapy with FASENRA. Reductions in corticosteroid dose, if appropriate, should be gradual and performed under the direct supervision of a physician. Reduction in corticosteroid dose may be associated with systemic withdrawal symptoms and/or unmask conditions previously suppressed by systemic corticosteroid therapy.
Parasitic (Helminth) Infection
It is unknown if FASENRA will influence a patient’s response against helminth infections. Treat patients with pre-existing helminth infections before initiating therapy with FASENRA. If patients become infected while receiving FASENRA and do not respond to anti-helminth treatment, discontinue FASENRA until infection resolves.
ADVERSE REACTIONS
The most common adverse reactions (incidence ≥ 5%) include headache and pharyngitis.
Injection site reactions (e.g., pain, erythema, pruritus, papule) occurred at a rate of 2.2% in patients treated with FASENRA compared with 1.9% in patients treated with placebo.
USE IN SPECIFIC POPULATIONS
A pregnancy exposure registry monitors pregnancy outcomes in women exposed to FASENRA during pregnancy. To enroll call 1-877-311-8972 or visit www.mothertobaby.org/Fasenra.
The data on pregnancy exposure from the clinical trials are insufficient to inform on drug-associated risk. Monoclonal antibodies such as benralizumab are transported across the placenta during the third trimester of pregnancy; therefore, potential effects on a fetus are likely to be greater during the third trimester of pregnancy.
FASENRA is indicated for the add-on maintenance treatment of patients with severe asthma aged 6 years and older, and with an eosinophilic phenotype.
- FASENRA is not indicated for treatment of other eosinophilic conditions
- FASENRA is not indicated for the relief of acute bronchospasm or status asthmaticus
Please read full Prescribing Information , including Patient Information and Instructions for Use .
You may report side effects related to AstraZeneca products .
TATE Phase III Trial TATE was an open-label, Phase III trial evaluating the safety of FASENRA in children aged 6 to 11 years with severe eosinophilic asthma. The trial evaluated the PK, PD and safety of FASENRA administered subcutaneously in 28 children in the US and Japan aged 6 to 11 years, in addition to 2 patients aged 12 to 14 years in Japan with severe eosinophilic asthma over 48 weeks. 2
FASENRA met the primary endpoints in the trial, demonstrating PK, PD, and safety in children aged 6 to 11 years old with SEA were consistent with those seen in prior trials.
FASENRA is a monoclonal antibody that binds directly to IL-5 receptor alpha on eosinophils and attracts natural killer cells to induce rapid and near-complete depletion of blood and tissue eosinophils in most patients via apoptosis (programmed cell death). 5,6
FASENRA (benralizumab) is currently approved in more than 80 countries, including the US, EU, and Japan, and is approved for self-administration in the US, EU and other countries. 7-10 FASENRA has been prescribed to over 100,000 patients in the US. 11
FASENRA is in development for other diseases including chronic obstructive pulmonary disease, chronic rhinosinusitis with nasal polyps and hypereosinophilic syndrome. 12-14
FASENRA was developed by AstraZeneca and is in-licensed from BioWa, Inc., a wholly-owned subsidiary of Kyowa Kirin Co., Ltd., Japan.
AstraZeneca in Respiratory & Immunology Respiratory & Immunology, part of BioPharmaceuticals, is one of AstraZeneca’s main disease areas and is a key growth driver for the Company.
AstraZeneca is an established leader in respiratory care with a 50-year heritage. The Company aims to transform the treatment of asthma and COPD by focusing on earlier biology-led treatment, eliminating preventable asthma attacks, and removing COPD as a top-three leading cause of death. The Company’s early respiratory research is focused on emerging science involving immune mechanisms, lung damage and abnormal cell-repair processes in disease and neuronal dysfunction.
With common pathways and underlying disease drivers across respiratory and immunology, AstraZeneca is following the science from chronic lung diseases to immunology-driven disease areas. The Company’s growing presence in immunology is focused on five mid- to late-stage franchises with multi-disease potential, in areas including rheumatology (including systemic lupus erythematosus), dermatology, gastroenterology, and systemic eosinophilic-driven diseases. AstraZeneca’s ambition in Respiratory & Immunology is to achieve disease modification and durable remission for millions of patients worldwide.
AstraZeneca AstraZeneca is a global, science-led biopharmaceutical company that focuses on the discovery, development, and commercialization of prescription medicines in Oncology, Rare Diseases, and BioPharmaceuticals, including Cardiovascular, Renal & Metabolism, and Respiratory & Immunology. Based in Cambridge, UK, AstraZeneca operates in over 100 countries and its innovative medicines are used by millions of patients worldwide. Please visit www.astrazeneca-us.com and follow us on social media @AstraZeneca .
Brendan McEvoy +1 302 885 2677 Jillian Gonzales +1 302 885 2677
US Media Mailbox: [email protected]
- FASENRA US prescribing information. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/761070Orig1s020correctedlbl.pdf . [Last accessed April 2024].
- Wedner HJ, Fujisawa T, Guilbert TW, et al on behalf of the TATE Investigators. Benralizumab in children with severe eosinophilic asthma: pharmacokinetics and long-term safety (TATE study). Pediatri Allergy Immunol 2024;35:e14092.
- World Health Organization. Asthma. Available at: https://www.who.int/news-room/fact-sheets/detail/asthma. [Last accessed April 2024].
- Castagnoli R, Marseglia A, Brambilla I, Marseglia GL, Licari A. Severe uncontrolled asthma in children: practical approach on diagnosis and management. Minerva Pediatr . 2020;72(3):196-205.
- Kolbeck R, et al . MEDI-563, a humanized anti-IL-5 receptor a mAb with enhanced antibody-dependent cell-mediated cytotoxicity function. J Allergy Clin Immunol . 2010;125:1344-1353.e2.
- Pham TH, et al . Reductions in eosinophil biomarkers by benralizumab in patients with asthma. Respir Med . 2016;111:21-29.
- AstraZeneca news release. Available at: https://www.astrazeneca.com/media-centre/press-releases/2019/fasenra-approved-in-the-us-for-self-administration-in-a-new-pre-filled-auto-injector-the-fasenra-pen-04102019.html . [Last accessed: April 2024].
- AstraZeneca news release. Available at: https://www.astrazeneca.com/media-centre/press-releases/2019/fasenra-receives-positive-eu-chmp-opinion-for-self-administration-and-the-new-fasenra-pen-a-pre-filled-single-use-auto-injector-01072019.html . [Last accessed: April 2024].
- AstraZeneca Annual Report 2023. Available at: https://www.astrazeneca.com/content/dam/az/Investor_Relations/annual-report-2023/pdf/AstraZeneca_AR_2023.pdf . [Last accessed: April 2024].
- AstraZeneca news release. Fasenra met the primary endpoint in the MANDARA Phase III trial in eosinophilic granulomatosis with polyangiitis (EGPA). Available at: https://www.astrazeneca.com/media-centre/press-releases/2023/fasenra-phase-iii-egpa-trial-met-primary-endpoint.html#:~:text=Positive%20high%2Dlevel%20results%20from,EGPA)%20who%20were%20receiving%20oral . [Last accessed: April 2024].
- AstraZeneca plc. FY and Q4 2023 Results. Conference call and webcast for investors and analysts. Available at: https://www.astrazeneca.com/media-centre/press-releases/2024/full-year-and-q4-2023-results.html . [Last accessed April 2024].
- Clinicaltrials.gov. Efficacy and Safety of Benralizumab in Moderate to Very Severe Chronic Obstructive Pulmonary Disease (COPD) With a History of Frequent Exacerbations (RESOLUTE). Available from: https://clinicaltrials.gov/ct2/show/NCT04053634 . [Last accessed: April 2024].
- Clinicaltrials.gov. Efficacy and Safety Study of Benralizumab in Patient With Eosinophilic Chronic Rhinosinusitis With Nasal Polyps (ORCHID). Available at: https://clinicaltrials.gov/ct2/show/NCT04157335 . [Last accessed: April 2024].
- Clinicaltrials.gov. A Phase 3 Study to Evaluate the Efficacy and Safety of Benralizumab in Patients With Hypereosinophilic Syndrome (HES) (NATRON). Available from: https://clinicaltrials.gov/ct2/show/NCT04191304 . [Last Accessed: April 2024].
You are now leaving AstraZeneca-us.com
You have selected a link that will take you to a site maintained by a third party who is solely responsible for its contents.
AstraZeneca provides this link as a service to website visitors. AstraZeneca is not responsible for the privacy policy of any third party websites. We encourage you to read the privacy policy of every website you visit.
Click ‘cancel’ to return to AstraZeneca’s site or ‘continue’ to proceed.
Important notice for users You are about to access AstraZeneca historic archive material. Any reference in these archives to AstraZeneca products or their uses may not reflect current medical knowledge and should not be used as a source of information on the present product label, efficacy data or safety data. Please refer to your approved national product label (SmPC) for current product information. I have read this warning and will not be using any of the contained product information for clinical purposes.
Asthma: Evidence-Based Pharmacological Treatment Research Paper
Pathophysiology, genomic issues, literature review, data collection, clinical guideline for asthma management, treatment approaches, follow-up treatment and referrals.
Asthma is typically defined as a chronic disease that affects the patient’s airways, impeding the process of breathing (National Heart, Lung, and Blood Institute, 2014). The understanding of how asthma is developed and by what factors it is triggered has been altered significantly over the past few years. At present, the pathophysiology of the disease is rather intricate since several conditions have been identified as the leading causes of asthma.
For instance, “Bronchospasms, edema, excessive mucus, and epithelial and muscle damage” (Lynn & Kushto-Reese, 2015, p. 49) are typically viewed as the key contributors to asthma development in patients (Lynn & Kushto-Reese, 2015). Each of the conditions listed above causes the development of bronchoconstriction with bronchospasms, therefore, making airways narrow (Lynn & Kushto-Reese, 2015). As a result, the premises for an asthma attack are created (see Appendix A).
It should be borne in mind, though, that the degree of disease variation in patients hinges on not the symptoms but the age thereof, as a recent study indicates (Kudo, Ishigatsubo, & Aoki, 2013). For instance, in children under 6, the development of the disease is typically preceded by the asthma-like symptoms that manifest themselves roughly at the age of three (Centers for Disease Control and Prevention, 2017).
A deficit in lung function can be observed in the identified group of patients at the age of 11-16. The force expiratory volume, however, remains consistent. The observations mentioned above are strikingly different from the ones carried out in a group of children that developed asthma after the age of three. Particularly, the specified patients did not show the propensity toward the development of lung function deficit (National Heart, Lung, and Blood Institute, 2014).
Asthma is currently viewed as a disease induced by both environmental and genetic factors. Therefore, understanding the genomic characterization of the disease is crucial to its management. Genome-wide association studies (GWASs) carried out lately indicate that the PGAP3 gene, or PERLD1, defines the development of asthma in patients (Li et al., 2013).
It should be noted, though, that there are uncertainties in the current research on the subject of genomes and asthma. For instance, a recent analysis of the issue shows that, while PGAP3 has a tangible effect on the development of allergic reactions to specific elements, it is GSDMB that determines the propensity toward the disease development in patients: “STARD3/PGAP3 may act on the allergic component of asthma while the GSDMB/ORMDL3/GSDMA locus may mediate its effect by a shared mechanism acting on most forms of asthma” (Lavoie-Charland, Berube, Boulet, & Bosse, 2016, p. 909). Therefore, there are at least two genomes that possibly cause the development of asthma in patients (Lavoie-Charland et al., 2016).
A whole-blood expression quantitative trait loci (eQTL) used in the research conducted by aaa showed that the functional variants of 17q12-21 are also associated with the development of allergic reactions toward a set of specific irritants (Andiappan et al., 2016). It is remarkable, though, that 17q12-21 affects the development of allergic asthma yet not rhinitis (Andiappan et al., 2016). Particularly, there is a connection between the genes at 17q12-21 and the polymorphism rs8076131 (Andiappan et al., 2016).
It should be borne in mind, though, that rs8076131 is not the only polymorphism that is associated with asthma. The article written by Schedel et al. (2015) shows that rs4065275, as well as the group of polymorphisms within ORMDL3, in general, is linked directly to the TH2 cytokines levels and, therefore, affects the susceptibility to asthma, especially among children (Schedel et al., 2015). However, further studies of the identified connection, as well as how the genomes in question affect the development of the disease, is required.
Seeing that asthma cannot be cured completely, the current approaches to managing it are aimed primarily at reducing the symptoms (e.g., coughing, shortness of breath, etc.) and improving the patients’ quality of life. At present, active engagement of patients and, if needed, their parents or legal guardians in the process of managing the disorder is viewed as a necessity. For the people that are capable of taking care of themselves, the promotion of patient independence is considered a crucial step in addressing the adverse effects of asthma successfully (Jain et al., 2014).
Two primary types of asthma treatment are utilized presently. These are short-term relievers (bronchodilators, particularly, dry powder inhalers and nebulizers (Daley-Yates, Mehta, Chan, Despa, & Louey, 2014)) and long-term disease control medications (Loymans et al., 2014).
Among the former, corticosteroids that are administered orally or intravenously are typically listed. Short-term relievers, which include bronchodilators, are used for three primary goals, i.e., the relaxation of the smooth muscle by stimulating beta-2 receptors, prevention of the cholinergic nerves activation, and cessation of the phosphodiesterase (PDE) enzymes’ functioning so that the further dilation of the bronchial airways could be possible (Normansell & Welsh, 2015). Long-term medication, in turn, also include corticosteroids (e.g., budesonide, beclomethasone, fluticasone, etc.). Apart from corticosteroids, leukotriene modifiers (zileuton, montelukast, etc.) are used to reduce the threat of an asthma attack (Lajqi, Ilazi, Kastrati, & Islami, 2015).
To gather the essential information about asthma, its development, and treatment, as well as other issues associated with the disease, an overview of the recent studies and the databases that contained the general information about asthma was required. Therefore, a literature review was used as the primary approach to data collection and its further interpretation so that it could be utilized in the study. As a result, a comprehensive review of the available information became possible.
Searching for the relevant data required using specific keywords. It should be noted, though, that the information about different aspects of the disease had to be utilized in the research. Therefore, different sets of keywords were used for each of the search processes.
Table 1. Keywords used in the Search Process.
In the course of the search, ResearchGate and NCBI were used as the key databases for locating the essential information. The two resources were selected because they provide academic, peer-reviewed, and credible articles. Less trustworthy databases were discarded.
Electronic Clinical Tools
No electronic clinical tools were utilized in the research. Since there was no need for a quantitative study, including the information from HER provided by local healthcare facilities did not seem necessary. Instead, an all-embracive analysis of the articles obtained from scholarly databases was conducted.
At present, the U.S. standards for asthma management are defined by the guide provided by the NCBI in 2007 (National Heart, Lung, and Blood Institute, 2007). Even though the identified resource is comparatively old, it remains the basis for the diagnosis and management of asthma. The guide provides detailed information about the nature of the problem, its pathophysiology, and the available treatment methods.
The guide places an especially strong emphasis on the importance of patient education as the path to successful management of the disease: “Asthma self-management education should be integrated into all aspects of asthma care, and it requires repetition and reinforcement” (National Heart, Lung, and Blood Institute, 2007, p. 93). The identified guideline should be improved and followed closely nowadays by introducing social media into the process of patient education as the means of keeping the target population updated on the latest information and promoting cooperation between a nurse and the community.
For instance, engaging asthma patients in a dialogue by encouraging them to participate in Facebook discussions of the problem and responding to the information posted by the healthcare services will be a crucial step on the way to improving the management of asthma. Furthermore, a range of myths about asthma will be debunked. As a result, patients will feel empowered for developing independence in identifying asthma-related symptoms and searching for the assistance of healthcare experts (Panzera et al., 2013).
As stressed above, the complete treatment of asthma is impossible at present; instead, its consistent management is used to improve the quality of the patient’s life (Jain et al., 2014). Therefore, an approach to handling the disorder in a long-term perspective needs to be considered. Furthermore, one must keep in mind that there are treatment strategies for three stages of asthma development (i.e., the so-called green, yellow, and red zones) (Yin et al., 2012). For this purpose, the stepwise approach must be used (Inoue et al., 2017). A common treatment framework implies carrying out the following steps:
- Identifying the severity of the problem (i.e., either a green, yellow, or red zone stage);
- Providing the patient with a quick-relief medication, if necessary (Albuterol, Levalbuterol, Metaproterenol, or Terbutaline) (U.S. National Library of Medicine, 2017);
- Expectorating the secretions of the patient so that the further aggravation of the problem could be avoided;
- Maintaining the airway clean so that the necessary medications could be administered to the patient;
- Continuing to provide long-term medications such as dry powder inhalers and nebulizers (1-2 puffs a day) if the short-term medication is efficient;
- Educating the patient about the symptoms, the necessity to avoid the allergens, the need to use the provided medications (1-2 puffs of nebulizers per day), etc. (Inoue et al., 2017).
Alternative treatment approaches may involve the use of fluid therapy (in case of a patient experiences dehydration) and pharmacologic therapy, which allows determining the patient’s response to medications (Bendjelid, Rex, Scheeren, & Critchley, 2015). However, the plan outlined above creates premises for all-embracive management of asthma and, thus, should be viewed as the superior one.
Best Approach: Selection and Rationale
As stressed above, the framework involving the classification of the asthma attack severity and the identification of the patient’s age (i.e., the stepwise approach) allows for the best results. The reason for choosing the specified strategy as the most efficient one is that it helps determine the patients’ needs fast and accurately. As a result, the chances for a patient outcome increase greatly (Inoue et al., 2017).
The use of fluid therapy, while also being quite effective, cannot be seen as an independent strategy for managing asthma since it does not include the comprehensive model that can be used to assist any asthma patient. Instead, it can only be utilized in specific cases of certain asthma severity. Therefore, it cannot be viewed as the ultimate tool for handling asthma cases (Bendjelid et al., 2015).
Consequently, the stepwise approach must be considered the most effective tool. It produces an immediate result and contributes to a massive improvement in the patient’s condition. Furthermore, the identified framework helps monitor asthma in patients of any age, from newborns to adults. The fact that the strategy allows for a minimum of adverse effects should also be considered one of its primary advantages (Inoue et al., 2017). Thus, it should be used as the basis for handling asthma cases.
The further management of the case involves educating the patient about asthma and its management. Particularly, independence must be encouraged. The patient must be able to identify the symptoms that may pose a threat to their well-being and contact the healthcare services. Patient referrals must be considered a necessity in case of an asthma episode exacerbation. Subsequent tests carried out by an immunologist are obligatory for the further analysis of the problem and the identification of the possible treatment options. Furthermore, patient education must follow the treatment process so that further instances of asthma could be addressed efficiently.
Andiappan, A. K., Sio, Y. Y., Lee, B., Suri, B. K., Matta, S. A., Lum, J., … Chew, F. T. (2016). Functional variants of 17q12-21 are associated with allergic asthma but not allergic rhinitis. Journal of Allergy and Clinical Immunology, 137 (3), 758-766. Web.
Asthma: Practice essentials, background, anatomy . (2017). Web.
Bendjelid, K., Rex, S., Scheeren, T., & Critchley, L. (2015). Year in review in journal of clinical monitoring and computing 2014: Cardiovascular and hemodynamic monitoring. Journal of Clinical Monitoring and Computing, 29 (2), 203-207. Web.
Centers for Disease Control and Prevention. (2017). Learn how to control asthma . Web.
Daley-Yates, P. T., Mehta, R., Chan, R. H., Despa, S. X., & Louey, M. D. (2014). Pharmacokinetics and pharmacodynamics of fluticasone propionate and salmeterol delivered as a combination dry powder from a capsule-based inhaler and a multidose inhaler in asthma and COPD patients. Journal of Aerosol Medicine and Pulmonary Drug Delivery, 27 (4), 279-289. Web.
Inoue, H., Nagase, T., Morita, S., Yoshida, A., Jinnai, T., & Ichinose, M. (2017). Prevalence and characteristics of asthma–COPD overlap syndrome identified by a stepwise approach. International Journal of Chronic Obstructive Pulmonary Disease, 12 (1), 1803-1810. Web.
Jain, V. V., Allison, R., Beck, S. J., Jain, R., Mills, P. K., Mccurley, J. W., … Peterson, M. W. (2014). Impact of an integrated disease management program in reducing exacerbations in patients with severe asthma and COPD. Respiratory Medicine, 108 (12), 1794-1800. Web.
Kudo, M., Ishigatsubo, Y., & Aoki, I. (2013). Pathology of asthma. Frontiers in Microbiology, 4 (263), 1-16. Web.
Lajqi, N., Ilazi, A., Kastrati, B., & Islami, H. (2015). Comparison of Glucocorticoid (Budesonide) and Antileukotriene (Montelukast) effect in patients with bronchial asthma determined with body plethysmography. Acta Informatica Medica, 23 (6), 347-351. Web.
Lavoie-Charland, E., Berube, J. C., Boulet, L. P., & Bosse, Y. (2016). Asthma susceptibility variants are more strongly associated with clinically similar subgroups. Journal of Asthma, 53 (9), 907-13. Web.
Li, L., Kabesch, M., Bouzigon, E., Demenais, F., Farrall, M., Moffatt, M. F., … Liang, L. (2013). Using eQTL weights to improve power for genome-wide association studies: A genetic study of childhood asthma. Frontiers in Genetics, 4 (103), 1-14. Web.
Loymans, R. J., Gemperli, A., Cohen, J., Rubinstein, S. M., Sterk, P. J., Reddel, H. K., … Riet, G. T. (2014). Comparative effectiveness of long term drug treatment strategies to prevent asthma exacerbations: Network meta-analysis. BMJ, 348 (1), 1-16. Web.
Lynn, S. J., & Kushto-Reese, K. (2015). Understanding asthma pathophysiology, diagnosis, and management Learn about new research findings and current treatment strategies for this common disorder. American Nurse Today, 10 (7), 49-51.
National Heart, Lung, and Blood Institute. (2007). Guidelines for the diagnosis and management of asthma (EPR-3): Full report . Web.
National Heart, Lung, and Blood Institute. (2014). What is asthma? Web.
Normansell, R., & Welsh, E. (2015). Asthma can take over your life but having the right support makes that easier to deal with. Informing research priorities by exploring the barriers and facilitators to asthma control: A qualitative analysis of survey data. Asthma Research and Practice, 1 (1), 11-21. Web.
Panzera, A. D., Schneider, T. K., Martinasek, M. P., Lindenberger, J. H., Couluris, M., Bryant, C. A., & Mcdermott, R. J. (2013). Adolescent asthma self-management: Patient and parent-caregiver perspectives on using social media to improve care. Journal of School Health, 83 (12), 921-930. Web.
Schedel, M., M., S., Gaertner, V. D., Toncheva, A. A., Depner, M., Binia, A., … Kabesch, M. (2015). Polymorphisms related to ORMDL3 are associated with asthma susceptibility, alterations in transcriptional regulation of ORMDL3, and changes in TH2 cytokine levels. Journal of Allergy and Clinical Immunology, 136 (4), 758-768. Web.
U.S. National Library of Medicine. (2017). Asthma – Quick-relief drugs . Web.
Yin, H. S., Gupta, R. S., Tomopoulos, S., Wolf, M. S., Mendelsohn, A. L., Antler, L., … Dreyer, B. P. (2012). Readability, suitability, and characteristics of asthma action plans: Examination of factors that may impair understanding. Pediatrics, 131 (1), 118-128. Web.
- Chicago (A-D)
- Chicago (N-B)
IvyPanda. (2024, January 23). Asthma: Evidence-Based Pharmacological Treatment. https://ivypanda.com/essays/asthma-evidence-based-pharmacological-treatment/
"Asthma: Evidence-Based Pharmacological Treatment." IvyPanda , 23 Jan. 2024, ivypanda.com/essays/asthma-evidence-based-pharmacological-treatment/.
IvyPanda . (2024) 'Asthma: Evidence-Based Pharmacological Treatment'. 23 January.
IvyPanda . 2024. "Asthma: Evidence-Based Pharmacological Treatment." January 23, 2024. https://ivypanda.com/essays/asthma-evidence-based-pharmacological-treatment/.
1. IvyPanda . "Asthma: Evidence-Based Pharmacological Treatment." January 23, 2024. https://ivypanda.com/essays/asthma-evidence-based-pharmacological-treatment/.
Bibliography
IvyPanda . "Asthma: Evidence-Based Pharmacological Treatment." January 23, 2024. https://ivypanda.com/essays/asthma-evidence-based-pharmacological-treatment/.
- Corticosteroids and Inhalants in Asthma
- Use of Scientific Method in Asthma and Allergic Reactions Study
- Asthma: Pathophysiology, Symptoms, and Manifestations
- Treatment of Asthma in Australia
- Asthma: Causes and Treatment
- Asthma: Pathophysiology, Etiology, Diagnosis, and Complications
- Allergic Diseases and the Hygiene Hypothesis
- Biological Basis of Asthma and Allergic Disease
- Asthma and Stepwise Management
- Asthma Pathophysiology and Genetic Predisposition
- Prescription Drug Abuse in the United States
- Hypokalemia and Prescribed Drug Interactions
- Nursing Care Priorities: Juan Carlos' Case
- The Rise of the Indian Pharmaceutical Industry
- Valproic Acid as a Psychopharmacological Treatment
Ozempic Hurts the Fight Against Eating Disorders
I t’s impossible to escape the soaring popularity of Ozempic and similar drugs these days—daily headlines, celebrity “success” stories, and apparent ease in procuring prescriptions (even Costco sells them now) abound. But the cumulative effect of all of this has many experts in the eating disorder field worried about how this might affect their patients. This makes sense—even for those without eating disorders, these drugs can feel both triggering and enticing. After all, research tells us about 90% of women are dissatisfied with their bodies. This sounds like a quick fix.
Then, I started hearing reports—first anecdotal, then published —that some doctors were prescribing weight loss drugs like Ozempic to their patients with eating disorders. As in, to help treat them.
As a journalist who has extensively researched the harms of eating disorders and the barriers to recovery—and as a woman who had suffered from eating disorders on and off for much of my own life—I thought I must have misunderstood. Yes, we as a society are in the midst of Ozempic Fever—and by “fever,” I’m referring to excitement, rather than a possible side effect of the drug (which it is). Researchers are continuing to find new potential applications for these drugs, initially developed to treat type 2 diabetes. In March, the FDA approved a new indication for the weight-loss drug Wegovy (which has the same active ingredient as Ozempic), allowing it to be used as a treatment to reduce the risk for heart attack and stroke. Ozempic, a diabetes drug, used off-label for weight loss, is also being studied to treat anxiety and depression , polycystic ovary syndrome, substance abuse, Alzheimer’s , and now—eating disorders.
Read More: Ozempic Exposed the Cracks in the Body Positivity Movement
It’s early days and research hasn’t yet caught up with the enthusiasm. But our cultural misunderstanding of eating disorders, even by well-meaning practitioners, could exacerbate the illnesses for those who suffer from them—and have dire consequences.
The new class of weight loss drugs mimics the body’s GLP-1 hormone , stimulating insulin production, and lowering blood sugar levels, helpful to those with type 2 diabetes. The drugs also curb appetite and slow the speed that food moves into the small intestine—you feel full more quickly and eat less. Many patients without eating disorders who take these drugs, have reported a reduction of “food noise” in their minds—referring to obsessive thoughts and preoccupation with food. (Though, as philosopher Kate Manne wisely posited in a recent New York Times piece , isn’t “food noise,” simply, hunger?)
For folks suffering from binge eating disorder (BED) or bulimia nervosa (BN), a drug that decreases appetite may seem to make sense. Both illnesses are characterized by eating large amounts of food, eating until uncomfortably full, and feeling distress around that (bulimia is distinguished by purging after a binge).
Binge eating often emerges as part of a cycle of restriction—dieting, fasting, or eliminating entire food groups—like carbs, for example. “Many people struggling with BED view the binge episodes as the problem and the restriction as something to strive for,” said Alexis Conason, a psychologist specializing in the treatment of binge eating disorder. “When people with BED take a GLP-1 medication that dampens their appetite, many are excited that they can be ‘better’ at restriction and consume very little throughout the day.” Subsequently, Conason adds, there is a dangerous potential for BED to then morph into anorexia, starving oneself with possibly life-threatening complications.
Eating disorders are complex illnesses that aren’t yet fully understood, even by experts in the field. Underneath the behaviors around food is often an intricate web of trauma, anxiety, and even genetic predisposition, all set against the backdrop of a culture that prizes thinness . Low weight is frequently (incorrectly) conflated with good health, and people in larger bodies are often subjected to bullying, negative stereotypes, and discrimination in the workplace .
Read More: Ozempic Gets the Oprah Treatment in a New TV Special
Emerging research strongly supports that for many, eating disorders are brain-based illnesses and in most cases, there exists a co-morbidity like anxiety, mood disorders, or substance abuse.
“GLP-1’s can’t help someone deal with their stress, anxiety, [and] trauma-history,” said psychologist Cynthia Bulik, one of the world’s leading eating disorder researchers, and Founding Director of the University of North Carolina Center of Excellence of Eating Disorders. “All of that background distress—fundamental distress that might be driving the BED in the first place—is temporarily bypassed by removing the desire to eat.”
Nearly 30 million Americans will have an eating disorder in their lifetime, but only about 6% of those are medically diagnosed as “underweight,” according to the National Association of Anorexia Nervosa and Associated Disorders. This means that a person may exhibit all of the diagnostic hallmarks of anorexia, for example, extreme restriction and even malnourishment, but still present as average weight or even overweight. They may even be told by a physician to lose weight, despite the fact that they are already going to dangerous extremes to chase that “goal.”
“We tend to think that everyone in a larger body with an eating disorder must have BED and everyone in a smaller body must have anorexia, but this couldn’t be further from the truth,” said Conason. “So many people with BED seek help in weight loss settings instead of seeking eating disorder treatment; many view the problem as their weight and think they need more help sticking to their diet” when in reality, an end to the restriction would more likely regulate their eating.
It’s much easier to get weight loss treatment than help for an eating disorder. There is no standard of care for eating disorders in this country and treatment is unregulated. While there are some promising, evidenced-based treatments (cognitive behavioral therapy for adults, and family-based treatment for children and teens), they don’t work for everyone. If a person is fortunate to be diagnosed and receive adequate treatment, relapses are common and full recovery can be elusive.
Further, these drugs are often intended to be taken for a person’s entire life. “When they go off the drug, or can’t access it due to supply problems, the urge to binge comes right back and they have not developed any psychological (or) behavioral skills to manage the urge,” Bulik told me. Just like with a diet, any lost weight will likely be regained when a person stops taking the drugs. Weight fluctuations, themselves ,may increase a person’s risk of chronic illnesses like type 2 diabetes, according to multiple studies.
“The focus on weight and erasing the desire to eat could indeed do harm,” cautioned Bulik. “The potential for abuse is high and will become higher with new preparations that don’t require an injection … Remember, these drugs are ‘for life.’ Stop them, and everything comes rushing back.”
The long-term side effects of GLP-1’s are not yet known. But the harms of eating disorders are: eating disorders have one of the highest mortality rates of any mental illness (second only to opioid overdose). People with eating disorders are more likely to attempt suicide, and during COVID-19, emergency room visits and inpatient admissions for eating disorders at pediatric hospitals skyrocketed, particularly for young women. According to the CDC, emergency room visits for 12-17 year old girls who suffer from eating disorders doubled during the pandemic. Those numbers, as shown by recent studies , have not returned to pre-pandemic levels.
An even greater concern is that the gaps in comprehensive care for eating disorders invite experimental, potentially harmful treatments and leave patients vulnerable. GLP-1’s may seem like a short-term “fix,” but they won’t graze the deeper issues nor will they diminish the eating disorder crisis in this country. And it is a crisis—every year, eating disorders cost the U.S. more than $65 billion .
I know too well that if a doctor advises their patient with an eating disorder “here’s something to make you eat less” most patients would happily oblige. That’s part of the pathology of the illness. It’s the eating disorder talking. Ideally, it wouldn’t be your doctor’s voice, too.
More Must-Reads From TIME
- The 100 Most Influential People of 2024
- The Revolution of Yulia Navalnaya
- 6 Compliments That Land Every Time
- Stop Looking for Your Forever Home
- If You're Dating Right Now , You're Brave: Column
- The AI That Could Heal a Divided Internet
- Fallout Is a Brilliant Model for the Future of Video Game Adaptations
- Want Weekly Recs on What to Watch, Read, and More? Sign Up for Worth Your Time
Contact us at [email protected]

IMAGES
VIDEO
COMMENTS
Asthma treatment is based on a stepwise approach. The management of the patient is control-based; that is, it involves an iterative cycle of assessment (e.g. symptoms, risk factors, etc.), adjustment of treatment (i.e. pharmacological, non-pharmacological and treatment of modifiable risk factors) and review of the response (e.g. symptoms, side ...
Oxygen therapy is also an essential immediately treatment for an acute asthma attack, write Christensen and Kockrow (2011). ... Asthma Essay With Conclusions. Asthma is one of the major chronic respiratory conditions which alter the respiratory function of the body. The World Health Organisation or WHO (2012) defines asthma as a chronic ...
Having asthma doesn't mean you have to be less active. Treatment can prevent asthma attacks and control symptoms during activity. Regular exercise can strengthen your heart and lungs, which helps relieve asthma symptoms. If you exercise in cold temperatures, wear a face mask to warm the air you breathe.
To manage symptoms and prevent exacerbations, asthma treatment typically consists of a combination of medications, including bronchodilators and corticosteroids. Prescription medication consists of bronchodilator, such as albuterol, to use as needed to relieve asthma symptoms the dispensation is through inhalation whenever there is experience ...
Follow this three-step approach to keep asthma symptoms under control and prevent asthma attacks. The goals of asthma treatment are to limit symptoms, prevent asthma attacks and avoid side effects of asthma medicines. The following three steps can help you take control of your asthma treatment. 1. Follow your asthma action plan.
This biologic drug is licensed as an add-on treatment for uncontrolled severe eosinophilic asthma in patients ≥18 years with ≥300 blood eosinophils/μl [ 56, 57 ]. A 30 mg dose of benralizumab is injected subcutaneously every 28 days for the first 3 administrations and afterwards every 56 days.
The prevalence of asthma in adults in the United States is approximately 7.7%. 1 It is one of the most common chronic, noncommunicable diseases in the country and worldwide. 1,2 Among U.S. adults ...
In these cases, the asthma does not respond to treatment, even with high dosages of medication or the correct use of inhalers. This type of asthma may affect 3.6% of people with the condition.
Asthma Essay. Sort By: Page 1 of 50 - About 500 essays. Decent Essays. Asthma And Its Effects On Asthma. 1066 Words; 5 Pages ... The child is dependent on parents' for initial management education of asthma treatment (Silva-Mendez & Barros, 2013, p.1002). The parents' beliefs about the adherence of medications have shown to have an influence.
Asthma is a disorder characterized by chronic airway inflammation, air way hypersensitivity to a variety of. stimuli, and airway obstruction. It is at least partially reversible, either ...
Asthma Diagnostics and Treatment. According to the Asthma and Allergy Foundation of America, some of the most common symptoms of asthma include cough, wheezing, shortness of breath, chest tightness, and fainting. Asthma: Pathophysiology, Symptoms, and Manifestations.
Asthma is a common chronic disease characterized by episodic or persistent respiratory symptoms and airflow limitation. Asthma treatment is based on a stepwise and control-based approach that involves an iterative cycle of assessment, adjustment of the treatment and review of the response aimed to minimize symptom burden and risk of exacerbations. Anti-inflammatory treatment is the mainstay of ...
Introduction. Asthma is an illness that disproportionately affects many adults and children globally. In 2019, 262 million people had asthma, causing 461 000 deaths (WHO, 2020). Scholars have done asthma-related research to provide information on causes, symptoms, therapies, and asthma mitigation. This study will describe asthma as a chronic ...
When it comes to writing an essay about asthma, there are countless interesting topics to explore. Some of the best asthma Essay Topics include: The impact of air pollution on asthma prevalence; The role of genetics in asthma development; The relationship between asthma and mental health; The effectiveness of alternative treatment methods for ...
Introduction Asthma is a complex heterogeneous disease, characterised by chronic airway inflammation and recurrent, epis - more. Essay Examples. Business; Education; Health; ... Asthma: Causes And Treatment Essay. Asthma In Children Aged 0-14 Essay. Asthma Triggers And Management Essay. Pathophysiology Of Asthma Essay.
Introduction. Asthma is the most common chronic respiratory disease affecting millions of people of all ages across the globe (Citation 1-6).The average global prevalence ranges between 5-10% (Citation 2).Traditionally, asthma diagnosis was based on the history and the response to a trial of various treatments, but emerging evidence shows that under the umbrella of asthma, several subtypes ...
Treatment of moderate persistent asthma requires increased doses of inhaled corticosteroids at 800-2,000 μ µg and long-acting β2‐adrenoceptor agonists. If a patient struggles with symptoms at nighttime, he or she should be given sustained-release theophylline or long-acting oral β2‐adrenoceptor agonists.
These causes affect how severe the asthma is and its treatment responsiveness. Before 12 years is mainly due to genetic causes, while onset after 12 is mainly due to environmental causes. There are many triggers that may cause asthma. Bronchial asthma triggers may include: Smoking and passive smoking, Perfumes, Allergens such as" food, dust ...
Apr 16, 2024, 10:47 pm. TUESDAY, April 16, 2024 (HealthDay News) -- The U.S. Food and Drug Administration has approved an additional indication for Fasenra (benralizumab) as an add-on maintenance treatment for patients aged 6 to 11 years with severe asthma and an eosinophilic phenotype. This indication was supported by evidence from the phase 3 ...
Additional indication for pediatric patients with severe eosinophilic asthma. AstraZeneca's FASENRA® (benralizumab) is now approved by the US Food and Drug Administration (FDA) for add-on maintenance treatment for patients with severe asthma aged 6 to 11 with an eosinophilic phenotype. 1 FASENRA was first approved in 2017 as an add-on maintenance for the treatment of severe eosinophilic ...
Asthma is typically defined as a chronic disease that affects the patient's airways, impeding the process of breathing (National Heart, Lung, and Blood Institute, 2014). The understanding of how asthma is developed and by what factors it is triggered has been altered significantly over the past few years. At present, the pathophysiology of ...
Ozempic, a diabetes drug, used off-label for weight loss, is also being studied to treat anxiety and depression, polycystic ovary syndrome, substance abuse, Alzheimer's, and now—eating ...