An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.


StatPearls [Internet].
Features, evaluation, and treatment of coronavirus (covid-19).
Marco Cascella ; Michael Rajnik ; Abdul Aleem ; Scott C. Dulebohn ; Raffaela Di Napoli .
Affiliations
Last Update: August 18, 2023 .
- Continuing Education Activity
Coronavirus disease 2019 (COVID-19) is a highly contagious infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). COVID-19 has had a catastrophic effect on the world, resulting in more than 6 million deaths worldwide. It has emerged as the most consequential global health crisis since the era of the influenza pandemic of 1918. As the virus mutates, treatment guidelines are altered to reflect the most efficacious therapies. This activity is a comprehensive review of the disease presentation, complications, and current guideline-recommended treatment options for managing this disease.
- Screen individuals based on exposure and symptom criteria to identify potential COVID-19 cases.
- Identify the clinical features and radiological findings expected in patients with COVID-19.
- Apply the recommended treatment options for patients with COVID-19.
- Create strategies with the interprofessional team for improving care coordination to care for patients with COVID-19 to help improve clinical outcomes.
- Introduction
Coronavirus disease 2019 (COVID-19) is a highly contagious viral illness caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). COVID-19 has had a catastrophic effect on the world, resulting in more than 6 million deaths worldwide. After the first cases of this predominantly respiratory viral illness were reported in Wuhan, Hubei Province, China, in late December 2019, SARS-CoV-2 rapidly disseminated worldwide. This compelled the World Health Organization (WHO) to declare it a global pandemic on March 11, 2020. [1]
Even though substantial progress in clinical research has led to a better understanding of SARS-CoV-2, many countries continue to have outbreaks of this viral illness. These outbreaks are primarily attributed to the emergence of mutant variants of the virus. Like other RNA viruses, SARS-CoV-2 adapts with genetic evolution and developing mutations. This results in mutant variants that may have different characteristics than their ancestral strains. Several variants of SARS-CoV-2 have been described during the course of this pandemic, among which only a few are considered variants of concern (VOCs). Based on the epidemiological update by the WHO, 5 SARS-CoV-2 VOCs have been identified since the beginning of the pandemic:
- Alpha (B.1.1.7): First variant of concern, which was described in the United Kingdom (UK) in late December 2020 [2]
- Beta (B.1.351) : First reported in South Africa in December 2020 [2]
- Gamma (P.1) : First reported in Brazil in early January 2021 [2]
- Delta (B.1.617.2): First reported in India in December 2020 [2]
- Omicron (B.1.1.529): First reported in South Africa in November 2021 [3]
Despite the unprecedented speed of vaccine development against the prevention of COVID-19 and robust global mass vaccination efforts, the emergence of new SARS-CoV-2 variants threatens to overturn the progress made in limiting the spread of this disease. This review aims to comprehensively describe the etiology, epidemiology, pathophysiology, and clinical features of COVID-19. This review also provides an overview of the different variants of SARS-CoV-2 and the guideline-recommended treatment (as of January 2023) for managing this disease.
Coronaviruses (CoVs) are positive-sense single-stranded RNA (+ssRNA) viruses with a crown-like appearance under an electron microscope ( coronam is the Latin term for crown) due to the presence of spike glycoproteins on the envelope. [1] The subfamily Orthocoronavirinae of the Coronaviridae family (order Nidovirales ) classifies into 4 genera of CoVs:
- Alphacoronavirus (alphaCoV)
- Betacoronavirus (betaCoV)
- Deltacoronavirus (deltaCoV)
- Gammacoronavirus (gammaCoV)
BetaCoV genus is further divided into 5 sub-genera or lineages. [4] Genomic characterization has shown that bats and rodents are the probable gene sources of alphaCoVs and betaCoVs. Avian species seem to be the source of deltaCoVs and gammaCoVs. CoVs have become significant pathogens of emerging respiratory disease outbreaks. Members of this large family of viruses can cause respiratory, enteric, hepatic, and neurological diseases in different animal species, including camels, cattle, cats, and bats.
These viruses can cross species barriers and infect humans as well. Seven human CoVs (HCoVs) capable of infecting humans have been identified. Some HCoVs were identified in the mid-1960s, while others were only detected in the new millennium. In general, estimates suggest that 2% of the population are healthy carriers of CoVs and that these viruses are responsible for about 5% to 10% of acute respiratory infections. [5]
- Common human CoVs : HCoV-OC43 and HCoV-HKU1 (betaCoVs of the A lineage), HCoV-229E, and HCoV-NL63 (alphaCoVs). These viruses can cause common colds and self-limiting upper respiratory tract infections in immunocompetent individuals. However, in immunocompromised and older patients, lower respiratory tract infections can occur due to these viruses.
- Other human CoVs : SARS-CoV and MERS-CoV (betaCoVs of the B and C lineage, respectively). These viruses are considered more virulent and capable of causing epidemics with respiratory and extra-respiratory manifestations of variable clinical severity. [1]
SARS-CoV-2 is a novel betaCoV belonging to the same subgenus as the severe acute respiratory syndrome coronavirus (SARS-CoV) and the Middle East Respiratory Syndrome Coronavirus (MERS-CoV), which have been previously implicated in SARS-CoV and MERS-CoV epidemics with mortality rates up to 10% and 35%, respectively. [6] It has a round or elliptic and often pleomorphic form and a diameter of approximately 60 to 140 nm. Like other CoVs, it is sensitive to ultraviolet rays and heat. [6]
The inactivation temperature of SARS-CoV-2 is being researched. A stainless steel surface held at an air temperature of 54.5°C (130 °F) results in the inactivation of 90% of SARS-CoV-2 in approximately 36 minutes. [7] It resists lower temperatures, even those below 0°C. However, lipid solvents can effectively inactivate these viruses, including ether (75%), ethanol, chlorine-containing disinfectant, peroxyacetic acid, and chloroform (except for chlorhexidine).
Although the origin of SARS-CoV-2 is currently unknown, it is widely postulated to have a zoonotic transmission. [1] Genomic analyses suggest that SARS-CoV-2 probably evolved from a strain found in bats. The genomic comparison between the human SARS-CoV-2 sequence and known animal coronaviruses revealed high homology (96%) between the SARS-CoV-2 and the betaCoV RaTG13 of bats ( Rhinolophus affinis ). [8] Similar to SARS and MERS, it has been hypothesized that SARS-CoV-2 advanced from bats to intermediate hosts, such as pangolins and minks, and then to humans. [9] [10]
SARS-CoV-2 Variants
A globally dominant D614G variant was eventually identified and associated with increased transmissibility but without the ability to cause severe illness. [11] Another variant was attributed to transmission from infected farmed mink in Denmark but was not associated with increased transmissibility. [10] Since then, multiple variants of SARS-CoV-2 have been described, of which a few are considered variants of concern (VOCs) due to their potential to cause enhanced transmissibility or virulence. The United States Centers for Disease Control and Prevention (CDC) and the WHO have independently established a classification system for distinguishing the emerging variants of SARS-CoV-2 into variants of concern(VOCs) and variants of interest(VOIs).
SARS-CoV-2 Variants of Concern (VOCs)
- Alpha (B.1.1.7 lineage)
- In late December 2020, the Alpha variant, or GRY (formerly GR/501Y.V1), was reported in the UK based on whole-genome sequencing of samples from patients who tested positive for SARS-CoV-2. [12] [13]
- The variant was also identified using a commercial assay characterized by the absence of the S gene (S-gene target failure, SGTF) in PCR samples. The B.1.1.7 variant includes 17 mutations in the viral genome. Of these, 8 mutations (Δ69-70 deletion, Δ144 deletion, N501Y, A570D, P681H, T716I, S982A, D1118H) are in the spike (S) protein. N501Y shows an increased affinity of the spike protein to ACE 2 receptors, enhancing the viral attachment and subsequent entry into host cells. [14] [15] [16]
- This alpha variant was reportedly 43% to 82% more transmissible, surpassing preexisting variants of SARS-CoV-2 to emerge as the dominant SARS-CoV-2 variant in the UK. [15]
- An initial matched case-control study reported no significant difference in the risk of hospitalization or associated mortality with the B.1.1.7 lineage variant compared to other existing variants. However, subsequent studies have reported that people infected with B.1.1.7 lineage variant had increased disease severity compared to those infected with other circulating variants. [17] [13]
- A large matched cohort study in the UK reported that the mortality hazard ratio of patients infected with the B.1.1.7 lineage variant was 1.64 (95% confidence interval 1.32 to 2.04, P<0.0001) compared to patients with previously circulating strains. [18]
- Another study reported that the B 1.1.7 variant was associated with increased mortality compared to other SARS-CoV-2 variants (HR= 1.61, 95% CI 1.42-1.82). [19] The risk of death was reportedly greater (adjusted hazard ratio 1.67, 95% CI 1.34-2.09) among individuals with confirmed B.1.1.7 infection compared to individuals with non-B.1.1.7 SARS-CoV-2. [20]
- Beta (B.1.351 lineage)
- The Beta variant, or GH501Y.V2 with multiple spike mutations, resulted in the second wave of COVID-19 infections and was first detected in South Africa in October 2020. [21]
- The B.1.351 variant includes 9 mutations (L18F, D80A, D215G, R246I, K417N, E484K, N501Y, D614G, and A701V) in the spike protein, of which 3 mutations (K417N, E484K, and N501Y) are located in the receptor binding domain (RBD) and increase its binding affinity for the ACE receptors. [22] [14] [23]
- SARS-CoV-2 501Y.V2 (B.1.351 lineage) was reported in the US at the end of January 2021.
- This variant had an increased risk of transmission and reduced neutralization by monoclonal antibody therapy, convalescent sera, and post-vaccination sera. [24]
- Gamma (P.1 lineage)
- The Gamma variant, or GR/501Y.V3 , was identified in December 2020 in Brazil and was first detected in the US in January 2021. [25]
- This B.1.1.28 variant harbors ten mutations in the spike protein (L18F, T20N, P26S, D138Y, R190S, H655Y, T1027I V1176, K417T, E484K, and N501Y). Three mutations (L18F, K417N, E484K) are located in the RBD, similar to the B.1.351 variant. [25]
- The Delta variant was initially identified in December 2020 in India and was responsible for the deadly second wave of COVID-19 infections in April 2021 in India. In the United States, this variant was first detected in March 2021. [2]
- The B.1.617.2 variant harbors ten mutations ( T19R, (G142D*), 156del, 157del, R158G, L452R, T478K, D614G, P681R, D950N) in the spike protein.
- The Omicron variant was first identified in South Africa on 23 November 2021 after an uptick in the number of cases of COVID-19. [26]
- Omicron was quickly recognized as a VOC due to more than 30 changes to the spike protein of the virus and the sharp rise in the number of cases observed in South Africa. [27] The reported mutations include T91 in the envelope, P13L, E31del, R32del, S33del, R203K, G204R in the nucleocapsid protein, D3G, Q19E, A63T in the matrix, N211del/L212I, Y145del, Y144del, Y143del, G142D, T95I, V70del, H69del, A67V in the N-terminal domain of the spike, Y505H, N501Y, Q498R, G496S, Q493R, E484A, T478K, S477N, G446S, N440K, K417N, S375F, S373P, S371L, G339D in the receptor-binding domain of the spike, D796Y in the fusion peptide of the spike, L981F, N969K, Q954H in the heptad repeat 1 of the spike as well as multiple other mutations in the non-structural proteins and spike protein. [28]
- Many subvariants of Omicron, such as BA.1, BA.2, BA.3, BA.4, and BA.5, have been identified. [3]
Transmission of SARS-CoV-2
- The primary mode of transmission of SARS-CoV-2 is via exposure to respiratory droplets carrying the infectious virus from close contact or direct transmission from presymptomatic, asymptomatic, or symptomatic individuals harboring the virus. [1]
- Airborne transmission with aerosol-generating procedures has also been implicated in the spread of COVID-19. Data implicating airborne transmission of SARS-CoV-2 in the absence of aerosol-generating procedures is present; however, this mode of transmission has not been universally acknowledged.
- Fomite transmission from contamination of inanimate surfaces with SARS-CoV-2 has been well characterized based on many studies reporting the viability of SARS-CoV-2 on various porous and nonporous surfaces. Under experimental conditions, SARS-CoV-2 was stable on stainless steel and plastic surfaces compared to copper and cardboard surfaces, with the viable virus being detected up to 72 hours after inoculating the surfaces with the virus. [29] The viable virus was isolated for up to 28 days at 20°C from nonporous surfaces such as glass and stainless steel. Conversely, recovery of SARS-CoV-2 on porous materials was reduced compared with nonporous surfaces. [30] In hospital settings, the SARS-CoV-2 has been detected on floors, computer mice, trash cans, sickbed handrails, and in the air (up to 4 meters from patients). [31] The Centers for Disease Control and Prevention (CDC) has stated that individuals can be infected with SARS-CoV-2 via contact with surfaces contaminated by the virus, but the risk is low and is not the main route of transmission of this virus.
- Epidemiologic data from several case studies have reported that patients with SARS-CoV-2 infection have the live virus in feces implying possible fecal-oral transmission. [32]
- A meta-analysis that included 936 neonates from mothers with COVID-19 showed vertical transmission is possible but occurs in a minority of cases. [33]
- Epidemiology
COVID-19 was the third leading cause of death in the United States (USA) in 2020 after heart disease and cancer, with approximately 375,000 deaths. [34]
Individuals of all ages are at risk of contracting this infection. However, patients aged ≥60 and patients with underlying medical comorbidities (obesity, cardiovascular disease, chronic kidney disease, diabetes, chronic lung disease, smoking, cancer, solid organ or hematopoietic stem cell transplant patients) have an increased risk of developing severe COVID-19 infection.
According to the CDC, age remains the strongest predictor of poor outcomes and severe illness in patients with COVID-19. Data from the National Vital Statistics System (NVSS) at CDC states that patients with COVID-19 aged 50 to 64 years have a 25 times higher risk of death when compared to adults infected with this illness and aged less than 30 years. In patients 65 to 74 years old, this risk increases to 60 times. In patients older than 85, the risk of death increases to 340 times. According to the CDC, these data include all deaths in the United States throughout the pandemic, from February 2020 to July 1, 2022, including deaths among unvaccinated individuals.
The percentage of COVID-19 patients requiring hospitalization was 6 times higher in those with preexisting medical conditions than those without medical conditions (45.4% vs. 7.6%) based on an analysis by Stokes et al. of confirmed cases reported to the CDC from January 22 to May 30, 2020. [35] The study also reported that the percentage of patients who succumbed to this illness was 12 times higher in those with preexisting medical conditions than those without (19.5% vs 1.6%). [35]
Data regarding the gender-based differences in COVID-19 suggests that male patients have a higher risk of severe illness and increased mortality due to COVID-19 compared to female patients. [36] [37] Results from a retrospective cohort study from March 1 to November 21, 2020, evaluating the mortality rate in 209 United States of America (USA) acute care hospitals that included 42604 patients with confirmed SARS-CoV-2 infection, reported a higher mortality rate in male patients (12.5%) compared to female patients (9.6%). [38]
Racial and ethnic minority groups have been reported to have a higher percentage of COVID-19-related hospitalizations than White patients based on a recent CDC analysis of hospitalizations from an extensive administrative database that included approximately 300,000 COVID-19 patients hospitalized from March 2020 to December 2020. This high percentage of COVID-19-related hospitalizations among racial and ethnic groups was driven by a higher risk of exposure to SARS-CoV-2 and an increased risk of developing severe COVID-19 disease. [39] A meta-analysis of 50 studies from USA and UK researchers noted that people of Black, Hispanic, and Asian ethnic minority groups are at increased risk of contracting and dying from COVID-19 infection. [40]
COVID-19-related death rates were the highest among Hispanic persons. [34] Another analysis by the CDC evaluating the risk of COVID-19 among sexual minority adults reported that underlying medical comorbidities which increase the risk of developing severe COVID-19 were more prevalent in sexual minority individuals than heterosexual individuals within the general population and within specific racial/ethnic groups. [41]
- Pathophysiology
Structurally and phylogenetically, SARS-CoV-2 is similar to SARS-CoV and MERS-CoV and is composed of 4 main structural proteins: spike (S), envelope (E) glycoprotein, nucleocapsid (N), and membrane (M) protein. It also contains 16 nonstructural proteins and 5-8 accessory proteins. [42]
The surface spike (S) glycoprotein, which resembles a crown, is located on the outer surface of the virion. It undergoes cleavage into an amino (N)-terminal S1 subunit, which facilitates the incorporation of the virus into the host cell. The carboxyl (C)-terminal S2 subunit contains a fusion peptide, a transmembrane domain, and a cytoplasmic domain responsible for virus-cell membrane fusion. [43] [44] The S1 subunit is further divided into a receptor-binding domain (RBD) and an N-terminal domain (NTD), which facilitates viral entry into the host cell and serves as a potential target for neutralization in response to antisera or vaccines . [45]
The RBD is a fundamental peptide in the pathogenesis of infection as it represents a binding site for the human angiotensin-converting enzyme 2 (ACE2) receptors. Inhibition of the renin-angiotensin-aldosterone system (RAAS) does not increase the risk of hospitalization for COVID-19 and severe disease. [46]
SARS-CoV-2 gains entry into the host cells by binding the SARS-CoV-2 spike or S protein (S1) to the ACE2 receptors in the respiratory epithelium. ACE2 receptors are also expressed by other organs such as the upper esophagus, enterocytes from the ileum, myocardial cells, proximal tubular cells of the kidney, and urothelial cells of the bladder. [47] The viral attachment process is followed by priming the spike protein S2 subunit by the host transmembrane serine protease 2 (TMPRSS2) that facilitates cell entry and subsequent viral replication. [48]
In the early phase of the infection, viral replication results in direct virus-mediated tissue damage. In the late phase, the infected host cells trigger an immune response by recruiting T lymphocytes, monocytes, and neutrophils. Cytokines such as tumor necrosis factor-α (TNF α), granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukin-1 (IL-1), interleukin-6 (IL-6), ), IL-1β, IL-8, IL-12 and interferon (IFN)-γ are released. In severe COVID-19 illness, a 'cytokine storm' is seen. This is due to the over-activation of the immune system and high levels of cytokines in circulation. This results in a local and systemic inflammatory response. [49] [50]
Effect of SARS-CoV-2 on the Respiratory System
Increased vascular permeability and subsequent development of pulmonary edema in patients with severe COVID-19 are explained by multiple mechanisms. [51] [52] [53] These mechanisms include:
- Endotheliitis as a result of direct viral injury and perivascular inflammation leading to microvascular and microthrombi deposition
- Dysregulation of RAAS due to increased binding of the virus to the ACE2 receptors
- Activation of the kallikrein-bradykinin pathway, the activation of which enhances vascular permeability
- Enhanced epithelial cell contraction causes swelling of cells and disturbance of intercellular junctions
- The binding of SARS-CoV-2 to the Toll-Like Receptor (TLR) induces the release of pro-IL-1β, which mediates lung inflammation until fibrosis . [54]
Effect of SARS-CoV-2 on Extrapulmonary Organ Systems
Although the respiratory system is the principal target for SARS-CoV-2, other major organ systems such as the gastrointestinal tract (GI), hepatobiliary, cardiovascular, renal, and central nervous systems may also be affected. SARS-CoV-2–induced organ dysfunction is likely due to a combination of mechanisms, such as direct viral toxicity, ischemic injury caused by vasculitis, thrombosis, immune dysregulation, and renin-angiotensin-aldosterone system (RAAS) dysregulation. [55]
Cardiac involvement in COVID-19 is common and likely multifactorial. ACE2 receptors exhibited by myocardial cells may cause direct cytotoxicity to the myocardium leading to myocarditis. Proinflammatory cytokines such as IL-6 can also lead to vascular inflammation, myocarditis, and cardiac arrhythmias. [56]
Acute coronary syndrome (ACS) is a well-recognized cardiac manifestation of COVID-19. It is likely due to multiple factors, including proinflammatory cytokines, worsening of preexisting severe coronary artery disease, coronary plaque destabilization, microthrombogenesis, and reduced coronary blood flow. [57]
SARS-CoV-2 has a significant effect on the hematological and hemostatic systems as well. The mechanism of leukopenia, one of the most common laboratory abnormalities encountered in COVID-19, is unknown. Several hypotheses have been postulated that include ACE 2 mediated lymphocyte destruction by direct invasion by the virus, lymphocyte apoptosis due to proinflammatory cytokines, and possible invasion of the virus in the lymphatic organs. [58]
Thrombocytopenia is common in COVID-19 and is likely due to multiple factors, including virus-mediated suppression of platelets, autoantibodies formation, and coagulation cascade activation, resulting in platelet consumption. [59]
Thrombocytopenia and neutrophilia are considered a hallmark of severe illness. [55] Although it is well known that COVID-19 is associated with a state of hypercoagulability, the exact mechanisms that lead to the activation of the coagulation system are unknown and likely attributed to the cytokine-induced inflammatory response. The pathogenesis of this associated hypercoagulability is multifactorial. The hypercoagulability is probably induced by direct viral-mediated damage or cytokine-induced injury of the vascular endothelium leading to the activation of platelets, monocytes, and macrophages, with increased expression of von Willebrand factor and Factor VIII that results in the generation of thrombin and formation of a fibrin clot. [59] [60]
Other mechanisms that have been proposed include possible mononuclear phagocyte-induced prothrombotic sequelae, derangements in the renin-angiotensin system (RAS) pathways, and complement-mediated microangiopathy. [59]
- History and Physical
Clinical Manifestations of COVID-19
- The median incubation period for SARS-CoV-2 is estimated to be 5.1 days, and most patients will develop symptoms within 11.5 days of infection. [61]
- The clinical spectrum of COVID-19 varies from asymptomatic or paucisymptomatic forms to clinical illness characterized by acute respiratory failure requiring mechanical ventilation, septic shock, and multiple organ failure.
- It is estimated that 17.9% to 33.3% of infected patients will remain asymptomatic. [62] [63]
- Most symptomatic patients present with fever, cough, and shortness of breath. Less common symptoms include sore throat, anosmia, dysgeusia, anorexia, nausea, malaise, myalgias, and diarrhea. Stokes et al. reported that among 373,883 confirmed symptomatic COVID-19 cases in the USA, 70% experienced fever, cough, and shortness of breath, 36% reported myalgia, and 34% reported headache. [35]
- A large meta-analysis evaluating clinicopathological characteristics of 8697 patients with COVID-19 in China reported laboratory abnormalities that included lymphopenia (47.6%), elevated C-reactive protein levels (65.9%), elevated cardiac enzymes (49.4%), and abnormal liver function tests (26.4%). Other laboratory abnormalities included leukopenia (23.5%), elevated D-dimer (20.4%), elevated erythrocyte sedimentation rate (20.4%), leukocytosis (9.9%), elevated procalcitonin (16.7%), and abnormal renal function (10.9%). [64]
- A meta-analysis of 212 published studies with 281,461 individuals from 11 countries/regions reported that severe disease course was noted in about 23% of the patients, with a mortality rate of about 6% in patients infected with COVID-19. [65]
- An elevated neutrophil-to-lymphocyte ratio (NLR), an elevated derived NLR ratio (d-NLR), and an elevated platelet-to-lymphocyte ratio indicate a cytokine-induced inflammatory storm. [66]
Based on the severity of the presenting illness, which includes clinical symptoms, laboratory and radiographic abnormalities, hemodynamics, and organ function, the National Institutes of Health (NIH) issued guidelines that classify COVID-19 into 5 distinct types.[ NIH COVID-19 Treatment Guidelines ]
- Asymptomatic or Presymptomatic Infection : Individuals with positive SARS-CoV-2 test without any clinical symptoms consistent with COVID-19.
- Mild illness : Individuals who have symptoms of COVID-19, such as fever, cough, sore throat, malaise, headache, muscle pain, nausea, vomiting, diarrhea, anosmia, or dysgeusia but without shortness of breath or abnormal chest imaging.
- Moderate illness : Individuals with clinical symptoms or radiologic evidence of lower respiratory tract disease and oxygen saturation (SpO 2 ) ≥94% on room air.
- Severe illness : Individuals who have SpO 2 less than 94% on room air, a ratio of partial pressure of arterial oxygen to fraction of inspired oxygen (PaO 2 /FiO 2 ) of less than 300, marked tachypnea with a respiratory frequency of greater than 30 breaths/min, or lung infiltrates that are greater than 50% of total lung volume.
- Critical illness : Individuals with acute respiratory failure, septic shock, or multiple organ dysfunction. Patients with severe COVID-19 illness may become critically ill with the development of acute respiratory distress syndrome (ARDS). This tends to occur approximately one week after the onset of symptoms.
ARDS is characterized by a severe new-onset respiratory failure or worsening of an already identified respiratory picture. The diagnosis requires bilateral opacities (lung infiltrates >50%), not fully explained by effusions or atelectasis. The Berlin definition classifies ARDS into 3 types based on the degree of hypoxia, with the reference parameter being PaO 2 /FiO 2 or P/F ratio: [67]
- Mild ARDS : 200 mm Hg <PaO 2 /FiO 2 ≤300 mm Hg in patients not receiving mechanical ventilation or in those managed through noninvasive ventilation (NIV) by using positive end-expiratory pressure (PEEP) or a continuous positive airway pressure (CPAP) ≥5 cm H2O.
- Moderate ARDS : 100 mm Hg <PaO 2 /FiO 2 ≤200 mm Hg
- Severe ARDS : PaO 2 /FiO 2 ≤100 mm Hg
When PaO 2 is unavailable, a ratio of SpO 2 /FiO 2 ≤315 suggests ARDS. A multicenter prospective observational study that analyzed 28-day mortality in mechanically ventilated patients with ARDS concluded that COVID-19 patients with ARDS had features similar to other ARDS cohorts, and the risk of 28-day mortality increased with ARDS severity. [68]
Extrapulmonary Manifestations
- Acute kidney injury (AKI) is the most frequently encountered extrapulmonary manifestation of COVID-19 and is associated with an increased mortality risk. [69] A large multicenter cohort study of hospitalized patients with COVID-19 that involved 5,449 patients admitted with COVID-19 reported that 1993 (36.6%) patients developed AKI during their hospitalization, of which 14.3% of patients required renal replacement therapy (RRT). [70]
- Myocardial injury manifesting as myocardial ischemia/infarction (MI) and myocarditis are well-recognized cardiac manifestations in patients with COVID-19. Single-center retrospective study analysis of 187 patients with confirmed COVID-19 reported that 27.8% of patients exhibited myocardial injury indicated by elevated troponin levels. The study also noted that patients with elevated troponin levels had more frequent malignant arrhythmias and a higher mechanical ventilation frequency than patients with normal troponin levels. [71] A meta-analysis of 198 published studies involving 159698 COVID-19 patients reported that acute myocardial injury and a high burden of pre-existing cardiovascular disease were significantly associated with higher mortality and ICU admission. [72]
- Lymphopenia is a common laboratory abnormality in most patients with COVID-19. Other laboratory abnormalities include thrombocytopenia, leukopenia, elevated ESR levels, C-reactive protein (CRP), lactate dehydrogenase (LDH), and leukocytosis.
- COVID-19 is also associated with a hypercoagulable state, evidenced by the high prevalence of venous thromboembolic events. COVID-19 is associated with markedly elevated D-dimer and fibrinogen levels and prolonged prothrombin time (PT) and partial thromboplastin time (aPTT). [71] [55]
- GI symptoms (such as diarrhea, nausea, vomiting), anorexia, and abdominal pain are common. A meta-analysis reported that the weighted pool prevalence of diarrhea was 12.4% (95% CI, 8.2% to 17.1%), nausea or vomiting was 9% (95% CI, 5.5% to 12.9%), loss of appetite was 22.3% (95% CI, 11.2% to 34.6%) and abdominal pain was 6.2% (95% CI, 2.6% to 10.3%). The study also reported that the mortality rate among patients with GI symptoms was similar to the overall mortality rate. [73] Cases of acute mesenteric ischemia and portal vein thrombosis have also been described. [74]
- An acute increase in aspartate transaminase (AST) and alanine transaminase (ALT) is noted in 14% to 53% of patients with COVID-19 infection. [75]
- Guillain-Barré syndrome (GBS) cases from Northern Italy have also been reported. [76] [77]
- Acral lesions resembling pseudo chilblains (40.4%) are the most common cutaneous manifestation noted in patients with COVID-19. [78]
- Other cutaneous manifestations include erythematous maculopapular rash (21.3%), vesicular rashes (13%), urticarial rashes (10.9%), vascular rashes (4%) resembling livedo or purpura, and erythema multiforme-like eruptions (3.7%). [78]
Diagnostic Testing in COVID-19
A nasopharyngeal swab for SARS-CoV-2 nucleic acid using a real-time PCR assay is the standard diagnostic test.[ NIH COVID-19 Treatment Guidelines ] Commercial PCR assays have been authorized by the USA Food and Drug Administration (FDA) for the qualitative detection of SARS-CoV-2 virus using specimens obtained from nasopharyngeal swabs as well as other sites such as oropharyngeal, anterior/mid-turbinate nasal swabs, nasopharyngeal aspirates, bronchoalveolar lavage (BAL) and saliva.
The sensitivity of PCR testing depends on multiple factors, including the specimen's adequacy, time from exposure, and specimen source. [79] However, the specificity of most commercial FDA-authorized SARS-CoV-2 PCR assays is nearly 100%, provided there is no cross-contamination during specimen processing. SARS-CoV-2 antigen tests are less sensitive but have a faster turnaround time than molecular PCR testing. [80]
Despite the numerous antibody tests designed to date, serologic testing has limitations in specificity and sensitivity, and results from different tests vary. According to the NIH guidelines, diagnosing acute SARS-CoV-2 infection based on serologic testing is not recommended. They also stated that there is insufficient evidence to recommend for or against using serologic testing to assess immunity, even if it is used to guide clinical decisions about COVID-19 vaccines/monoclonal antibodies.[ NIH COVID-19 Treatment Guidelines ]
Other Laboratory Assessment
- Complete blood count (CBC), a comprehensive metabolic panel (CMP) that includes renal and liver function testing, and a coagulation panel should be performed in all hospitalized patients.
- Additional tests, such as ESR, C-reactive protein (CRP), ferritin, lactate dehydrogenase, and procalcitonin, can be considered in hospitalized patients. However, their prognostic significance in COVID-19 is not clear.
- A D-dimer level is required as it guides the use of therapeutic versus prophylactic doses of anticoagulation.
Imaging ModalitiesThis s viral illness commonly manifests as pneumonia, so radiological imaging such as chest x-rays, lung ultrasounds, and chest computed tomography (CT) are often obtained. However, there are no guidelines regarding the timing and choice of pulmonary imaging in patients with COVID-19.
When obtained, the chest X-ray usually shows bilateral multifocal alveolar opacities. Pleural effusions can also be demonstrated. The most common CT chest findings in COVID-19 are multifocal bilateral ground glass opacities with consolidation changes, usually in a patchy peripheral distribution. [81]
Radiologic imaging is not a sensitive method for detecting this disease. A retrospective study of 64 patients with documented COVID-19 reported that 20% had no abnormalities on chest radiographs during the illness. [82] A chest CT is more sensitive than a radiograph but is not specific. No finding on radiographic imaging can completely rule in or rule out COVID-19 illness. Therefore the American College of Radiology (ACR) advises against the routine use of chest CT for screening or diagnosis of COVID-19.[ ACR Position Statement for Diagnosis of COVID-19 ]
- Treatment / Management
According to the National Institutes of Health (NIH), the 2 main processes driving the pathogenesis of COVID-19 include replication of the virus in the early phase of the illness and dysregulated immune/inflammatory response to SARS-CoV-2 that leads to systemic tissue damage in the later phase of the disease.[ NIH COVID-19 Treatment Guidelines ] The guidelines, therefore, advise antiviral medications to halt viral replication in the early phase of the illness and immunomodulators in the later phase.
Remdesivir is the only antiviral drug approved by the USA Food and Drug Administration (FDA) to treat COVID-19. Ritonavir-boosted nirmatrelvir, molnupiravir, and high-titer COVID-19 convalescent plasma have Emergency Use Authorizations (EUAs) for treating COVID-19. Tixagevimab 300 mg plus cilgavimab 300 mg monoclonal antibodies have received EUAs that allow them to be used as SARS-CoV-2 preexposure prophylaxis (PrEP) in certain patients.
Many other monoclonal antibodies had EUAs; however, as Omicron subvariants emerged, their EUAs were revoked as they were no longer effective.
The most recent NIH treatment guidelines for the management of COVID-19 illness (accessed on January 3rd, 2023) are outlined below:[ NIH COVID-19 Treatment Guidelines ]
Nonhospitalized Adults With Mild-to-Moderate COVID-19 Illness Who Do Not Require Supplemental Oxygen
- The NIH recommends against using dexamethasone or any other systemic corticosteroids in patients who are not hypoxic. [83]
- Ritonavir-boosted nirmatrelvir is a combination of oral protease inhibitors. It has been shown to reduce hospitalization and death when given to high-risk, unvaccinated, nonhospitalized patients. It must be given within 5 days of symptoms onset. [84]
- It is a strong cytochrome P450 inhibitor with many drug-drug interactions that must be carefully assessed.
- Some interactions can be managed by temporarily holding the medication, some may be managed with dose adjustment, but some may warrant the use of alternate COVID-19 therapy.
- Ritonavir-boosted nirmatrelvir is not recommended in patients with an estimated glomerular filtration rate (eGFR) of less than 30 mL/min.
- The recommended dose is nirmatrelvir 300 mg with ritonavir 100 mg orally twice daily for 5 days.
- This is a nucleotide analog that inhibits the SARS-CoV-2 RNA polymerase
- The recommended duration of therapy in this setting is 3 days.
- The recommended dose is 200 mg IV on day 1, followed by 100 mg IV for 2 more days.
- It is a mutagenic ribonucleoside antiviral agent.
- Fetal toxicity has been reported in animal studies with this agent. Due to the risk of genotoxicity with this agent, it is not recommended in pregnant patients.
- This agent should only be used if both therapies are unavailable or cannot be given.
- The NIH guidelines recommend against using anti-SARS-CoV-2 monoclonal antibodies (mAbs) for treating COVID-19 in this cohort because the Omicron subvariants are not susceptible to these agents.
- Adequate and close medical follow-up is recommended; however, the frequency and duration of follow-up depend on individual risk factors and the severity of their symptoms.
- Risk factors for progression to severe disease include advanced age and underlying medical conditions. The CDC maintains an updated list of medical conditions associated with a high risk of progression.
- Asthma
- Cerebrovascular disease
- Chronic kidney disease
- Bronchiectasis
- COPD (Chronic obstructive pulmonary disease)
- Interstitial lung disease
- Pulmonary embolism
- Pulmonary hypertension
- Nonalcoholic fatty liver disease
- Alcoholic liver disease
- Autoimmune hepatitis
- Cystic fibrosis
- Diabetes, type 1 and 2
- Heart conditions (such as heart failure, coronary artery disease, or cardiomyopathies)
- HIV (Human immunodeficiency virus)
- Mental health conditions such as mood disorders and Schizophrenia spectrum disorders
- Obesity (defined as body mass index (BMI) of greater than 30 kg/m 2 or greater than 95th percentile in children)
- Pregnancy and recent pregnancy
- Smoking, current and former
- Solid organ or blood stem cell transplantation
- Tuberculosis
- Use of corticosteroids or other immunosuppressive medications ( CDC: Underlying Medical Conditions Associated with Higher Risk )
Therapeutic Management of Hospitalized Adults With COVID-19 Who Do Not Require Oxygen
- If patients are hospitalized for reasons other than COVID-19 illness and are not on oxygen, their management is similar to nonhospitalized patients.
- If they are hospitalized for COVID-19 illness but do not require oxygen, the NIH advises against the use of dexamethasone or any other systemic corticosteroid.
- A prophylactic dose of anticoagulation should be given if there is no contraindication.
- If they are hospitalized for COVID-19 illness, do not require oxygen, but are at high risk of progression to severe disease, they should be treated with remdesivir.
- The benefit of remdesivir is greatest when given early, ideally within ten days of symptom onset.
- Remdesivir should be given for 5 days or until hospital discharge.
Therapeutic Management of Hospitalized Adults With COVID-19 Who Require Conventional Oxygen
- Conventional oxygen is defined as oxygen that is NOT high-flow nasal cannula, noninvasive mechanical ventilation, mechanical ventilation, or extracorporeal membrane oxygenation (ECMO)
- For most patients in this cohort, the recommended treatment is dexamethasone plus remdesivir.
- Dexamethasone dose is 6 mg IV or oral (PO) once daily for up to 10 days or until hospital discharge (dexamethasone should not be continued at discharge). [83]
- If the patient is on minimal oxygen, remdesivir monotherapy (without dexamethasone) should be used.
- If remdesivir cannot be obtained or given, dexamethasone monotherapy is recommended.
- If dexamethasone is unavailable, corticosteroids such as prednisone, methylprednisolone, or hydrocortisone may be used.
- If the patient is already receiving dexamethasone but has rapidly increasing oxygen needs and/or signs of systemic inflammation, oral baricitinib or intravenous (IV) tocilizumab should be added to the treatment regimen as these agents have been shown to improve outcomes in rapidly decompensating patients. [85]
- Alternate immunomodulatory agents for this cohort include oral tofacitinib and IV sarilumab. These agents should only be used if baricitinib and tocilizumab are not available.
- If the D-dimer level is above normal in this cohort of patients, they recommend therapeutic anticoagulation if the patient is not pregnant and has no increased risk of bleeding. Contraindications for therapeutic anticoagulation in these patients include a platelet count of less than 50 x10^9 /L, hemoglobin less than 8 g/dL, use of dual antiplatelet therapy, any significant bleeding within the past 30 days, a history of a bleeding disorder or an inherited or active acquired bleeding disorder.
- For pregnant patients, a prophylactic dose of anticoagulation is recommended.
Therapeutic Management of Hospitalized Adults With COVID-19 who Require High-flow Nasal Cannula (HFNC) or Noninvasive Mechanical Ventilation (NIV)
- A meta-analysis study evaluating the effectiveness of HFNC compared to conventional oxygen therapy and NIV before mechanical ventilation reported that HFNC, when used before mechanical ventilation, could improve the prognosis of patients compared to conventional oxygen therapy and NIV. [86] HFNC or NIV is associated with decreased dispersion of exhaled air, especially when used with good interface fitting, thus creating a low risk of nosocomial transmission of the infection. [87] However, these treatment modalities are associated with a greater risk of aerosolization and should be used in negative-pressure rooms. [88]
- According to the NIH, dexamethasone plus oral baricitinib or dexamethasone plus IV tocilizumab are the preferred treatment regimens in these patients.
- Alternate immunomodulatory agents for this cohort include oral tofacitinib and IV sarilumab.
- Dexamethasone monotherapy is recommended if baricitinib, tocilizumab, or sarilumab cannot be obtained/given.
- Clinicians may consider adding remdesivir to corticosteroid and immunomodulator combination regimens in immunocompromised patients who require HFNC or NIV ventilation; however, using remdesivir without immunomodulators is not recommended.
- A prophylactic dose of anticoagulation is recommended in these patients.
- If patients were started on a therapeutic dose of heparin while on conventional oxygen therapy, they should be switched to prophylactic dosing at this time unless they have another indication for full anticoagulation.
Therapeutic Management of Hospitalized Adults With COVID-19 who Require Mechanical Ventilation (MV)
- The management of this cohort is the same as those requiring HFNC or NIV, except that remdesivir is not recommended.
- Remdesivir is most effective earlier in the course of the disease and in patients not on mechanical ventilation or ECMO.
- According to the NIH, one study showed a slight trend toward an increase in mortality in patients who received remdesivir while on mechanical ventilation or ECMO. [89]
- With this data in mind, the NIH recommends against using remdesivir in patients receiving MV or ECMO; however, if the patient was started on remdesivir and progressed to requiring mechanical ventilation or ECMO, they recommended continuing remdesivir to complete the treatment course.
High-Titer COVID-19 Convalescent Plasma (CCP)
- The United States Food and Drug Administration (FDA) approved convalescent plasma therapy under a EUA for patients with severe life-threatening COVID-19. [90] [91] Data from multiple studies evaluating the use of convalescent plasma in life-threatening COVID-19 has generated mixed results. Data from 3 small randomized control trials showed no significant differences in clinical improvement or overall mortality in patients treated with convalescent plasma versus standard therapy. [92] [93] [94]
- According to the NIH, high-titer CCP is not recommended in immunocompetent individuals.
- However, the NIH states that some experts consider it appropriate for use in immunocompromised individuals. Therefore, the current NIH guidelines state that there is insufficient evidence for or against the use of high-titer CCP for treating COVID-19 in hospitalized or nonhospitalized patients who are immunocompromised.
Medications/Treatments That Should NOT Be Used for the Treatment of COVID-19 According to the Latest NIH Guidelines [ NIH COVID-19 Treatment Guidelines ]
- Chloroquine or hydroxychloroquine with or without azithromycin
- Lopinavir/ritonavir
- Azithromycin
- Doxycycline
- Fluvoxamine
- Inhaled corticosteroids
- Excess supplementation of vitamin C, vitamin D, and zinc
- Interferons alfa, beta, or lambda
- Nitazoxanide
- Bamlanivimab plus etesevimab
- Bebtelovimab
- Casirivimab plus imdevimab
Preexposure Prophylaxis for SARS-CoV-2 Infection
- According to the NIH guidelines, tixagevimab plus cilgavimab is authorized by the FDA for preexposure prophylaxis of SARS-CoV-2 in people who are not expected to mount an adequate immune response to COVID-19 vaccination; however, the prevalence of Omicron subvariants that are resistant to tixagevimab plus cilgavimab is noted to be increasing rapidly.
- In the absence of alternative options, the NIH still recommends tixagevimab 300 mg plus cilgavimab 300 mg at this time.
- Tixagevimab and cilgavimab are potent anti-spike neutralizing monoclonal antibodies obtained from antibodies isolated from B cells of patients infected with SARS-CoV-2 that have demonstrated neutralizing activity against SARS-CoV-2 virus by binding to nonoverlapping epitopes of the viral spike-protein RBD. [95] [96] [97]
- The EUA authorizes its use in adult and pediatric patients with no current evidence of SARS-CoV-2 infection and no recent exposure to SARS-CoV-2-positive individuals. They must be moderately or severely immunocompromised or be on immunosuppressive medications.
- Differential Diagnosis
The symptoms of the early stages of the disease are nonspecific. Differential diagnosis should include the possibility of a wide range of infectious and noninfectious respiratory disorders.
- Community-acquired bacterial pneumonia
- Viral pneumonia
- Influenza infection
- Aspiration pneumonia
- Pneumocystis jirovecii pneumonia
- Middle East respiratory syndrome (MERS)
- Avian influenza A (H7N9) viral infection
- Avian influenza A (H5N1) viral infection
- Pulmonary tuberculosis
The prognosis of COVID-19 depends on various factors, including the patient's age, the severity of illness at presentation, preexisting conditions, how quickly treatment can be implemented, and response to treatment. The WHO currently estimates the global case fatality rate for COVID-19 is 2.2%. Results from a European multicenter prospective cohort study that included 4000 critically ill patients with COVID-19 reported a 90-day mortality of 31%, with higher mortality noted in geriatric patients and patients with diabetes, obesity, and severe ARDS. [98]
- Complications
COVID-19 is a systemic viral illness based on its involvement in multiple major organ systems.
- Patients with advanced age and comorbid conditions such as obesity, diabetes mellitus, chronic lung disease, cardiovascular disease, chronic kidney disease, chronic liver disease, and neoplastic conditions are at risk of developing severe COVID-19 and its associated complications. The most common complication of severe COVID-19 illness is progressive or sudden clinical deterioration leading to acute respiratory failure and ARDS or multiorgan failure leading to death.
- Patients with COVID-19 illness are also at increased risk of developing prothrombotic complications such as pulmonary embolisms, myocardial infarctions, ischemic strokes, and arterial thrombosis. [55]
- Cardiovascular system involvement results in malignant arrhythmias, cardiomyopathy, and cardiogenic shock.
- GI complications such as bowel ischemia, transaminitis, gastrointestinal bleeding, pancreatitis, Ogilvie syndrome, mesenteric ischemia, and severe ileus are often noted in critically ill patients with COVID-19. [99]
- Acute renal failure is the most common extrapulmonary manifestation of COVID-19 and is associated with an increased mortality risk. [69]
- A meta-analysis study of 14 studies evaluating the prevalence of disseminated intravascular coagulation (DIC) in hospitalized patients with COVID-19 reported that DIC was observed in 3% (95%: 1%-5%, P <0.001) of the included patients. Additionally, DIC was noted to be associated with severe illness and was a poor prognostic indicator. [100]
- More recent data have emerged regarding prolonged symptoms in patients who have recovered from COVID-19 infection, termed "post-acute COVID-19 syndrome." A large cohort study of 1773 patients performed 6 months after hospitalization with COVID-19 revealed that most exhibited at least one persistent symptom: fatigue, muscle weakness, sleep difficulties, or anxiety. Patients with severe illness also had an increased risk of chronic lung issues. [101]
- A retrospective cohort study that included 236,379 patients reported substantial neurological (intracranial hemorrhage, ischemic stroke) and psychiatric morbidity (anxiety disorder, psychotic disorder) 6 months after being diagnosed with COVID-19. [102]
- Secondary invasive fungal infections such as COVID-19-associated pulmonary aspergillosis and rhino-cerebro-orbital mucormycosis have increasingly been reported as complications in patients recovering from COVID-19. Risk factors for developing secondary fungal infection include comorbid conditions such as uncontrolled diabetes, associated lymphopenia, and excessive use of corticosteroids.
- Deterrence and Patient Education
The NIH COVID-19 Treatment Guidelines recommend COVID-19 vaccination as soon as possible for all eligible individuals. The CDC’s Advisory Committee on Immunization Practices (AI) determines eligibility eligibility. Four vaccines are authorized or approved in the United States to prevent COVID-19. According to the NIH guidelines, the preferred vaccines include:[ NIH COVID-19 Treatment Guidelines ]
- mRNA vaccine BNT162b2 (Pfizer-BioNTech)
- mRNA-1273 (Moderna)
- Recombinant spike protein with matrix-M1 adjuvant vaccine NVX-CoV2373 (Novavax)
The adenovirus vector vaccine Ad26.COV2.S (Johnson & Johnson/Janssen) is less preferred due to its risk of serious adverse events.[ NIH COVID-19 Treatment Guidelines ]
A primary series of COVID-19 vaccination is recommended for everyone older than 6 months in the United States. Bivalent mRNA vaccines that protect against the original SARS-CoV-2 virus strain and Omicron subvariants are recommended at least 2 months after receiving the primary vaccine series or a booster dose.[ NIH COVID-19 Treatment Guidelines ]
- Enhancing Healthcare Team Outcomes
SARS-CoV-2 and its variants continue to cause significant morbidity and mortality worldwide. Prevention and management of this highly transmissible respiratory viral illness require a holistic and interprofessional approach that includes physicians' expertise across specialties, nurses, pharmacists, public health experts, and government authorities. There should be open communication among the clinical providers, pharmacists, and nursing staff while managing patients with COVID-19. Each team member should strive to keep abreast of the latest recommendations and guidelines and be free to speak up if they notice anything that does not comply with the latest tenets for managing COVID patients; there is no place for a hierarchy in communication that prohibits any team member from voicing their concerns. This open interprofessional approach will yield the best outcomes.
Clinical providers managing COVID-19 patients on the frontlines should keep themselves periodically updated with the latest clinical guidelines about diagnostic and therapeutic options available in managing COVID-19, especially considering the emergence of new SARS-CoV-2 variants, which could significantly impact morbidity and mortality. Continued viral surveillance of new variants is crucial at regular intervals with viral genomic sequencing, given the possibility that more highly transmissible, more virulent, and treatment-resistant variants could emerge that can have a more catastrophic effect on global health in addition to the current scenario. A multi-pronged approach involving interprofessional team members can improve patient care and outcomes for this potentially devastating disease and help the world end this pandemic.
- Review Questions
- Access free multiple choice questions on this topic.
- Comment on this article.
Covid 19, Corona Replication Contributed by Rohan Bir Singh, MD
Clinical Presentation of Patients with CoVID-19 Contributed by Rohan Bir Singh, MD; Made with Biorender.com
SARS- CoV 2 Structure Contributed by Rohan Bir Singh, MD; Made with Biorender.com
Transmission Cycle of SARS CoV 2 Contributed by Rohan Bir Singh, MD; Made with Biorender.com
Single-stranded RNA genome of SARS-CoV2 Contributed by Rohan Bir Singh, MD; Made with Biorender.com
Disclosure: Marco Cascella declares no relevant financial relationships with ineligible companies.
Disclosure: Michael Rajnik declares no relevant financial relationships with ineligible companies.
Disclosure: Abdul Aleem declares no relevant financial relationships with ineligible companies.
Disclosure: Scott Dulebohn declares no relevant financial relationships with ineligible companies.
Disclosure: Raffaela Di Napoli declares no relevant financial relationships with ineligible companies.
This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) ( http://creativecommons.org/licenses/by-nc-nd/4.0/ ), which permits others to distribute the work, provided that the article is not altered or used commercially. You are not required to obtain permission to distribute this article, provided that you credit the author and journal.
- Cite this Page Cascella M, Rajnik M, Aleem A, et al. Features, Evaluation, and Treatment of Coronavirus (COVID-19) [Updated 2023 Aug 18]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
In this Page
Bulk download.
- Bulk download StatPearls data from FTP
Related information
- PMC PubMed Central citations
- PubMed Links to PubMed
Similar articles in PubMed
- Emerging Variants of SARS-CoV-2 and Novel Therapeutics Against Coronavirus (COVID-19). [StatPearls. 2024] Emerging Variants of SARS-CoV-2 and Novel Therapeutics Against Coronavirus (COVID-19). Aleem A, Akbar Samad AB, Vaqar S. StatPearls. 2024 Jan
- Providing Access To Monoclonal Antibody Treatment Of Coronavirus (COVID-19) Patients In Rural And Underserved Areas. [StatPearls. 2024] Providing Access To Monoclonal Antibody Treatment Of Coronavirus (COVID-19) Patients In Rural And Underserved Areas. Wood DA, Aleem A, Davis D. StatPearls. 2024 Jan
- Review The Biological Functions and Clinical Significance of SARS-CoV-2 Variants of Corcern. [Front Med (Lausanne). 2022] Review The Biological Functions and Clinical Significance of SARS-CoV-2 Variants of Corcern. Akkız H. Front Med (Lausanne). 2022; 9:849217. Epub 2022 May 20.
- Review Molecular evolution of SARS-CoV-2 from December 2019 to August 2022. [J Med Virol. 2023] Review Molecular evolution of SARS-CoV-2 from December 2019 to August 2022. Wolf JM, Wolf LM, Bello GL, Maccari JG, Nasi LA. J Med Virol. 2023 Jan; 95(1):e28366.
- Unraveling the Dynamics of Omicron (BA.1, BA.2, and BA.5) Waves and Emergence of the Deltacton Variant: Genomic Epidemiology of the SARS-CoV-2 Epidemic in Cyprus (Oct 2021-Oct 2022). [Viruses. 2023] Unraveling the Dynamics of Omicron (BA.1, BA.2, and BA.5) Waves and Emergence of the Deltacton Variant: Genomic Epidemiology of the SARS-CoV-2 Epidemic in Cyprus (Oct 2021-Oct 2022). Chrysostomou AC, Vrancken B, Haralambous C, Alexandrou M, Gregoriou I, Ioannides M, Ioannou C, Kalakouta O, Karagiannis C, Marcou M, et al. Viruses. 2023 Sep 15; 15(9). Epub 2023 Sep 15.
Recent Activity
- Features, Evaluation, and Treatment of Coronavirus (COVID-19) - StatPearls Features, Evaluation, and Treatment of Coronavirus (COVID-19) - StatPearls
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers
- Research article
- Open access
- Published: 04 June 2021
Coronavirus disease (COVID-19) pandemic: an overview of systematic reviews
- Israel Júnior Borges do Nascimento 1 , 2 ,
- Dónal P. O’Mathúna 3 , 4 ,
- Thilo Caspar von Groote 5 ,
- Hebatullah Mohamed Abdulazeem 6 ,
- Ishanka Weerasekara 7 , 8 ,
- Ana Marusic 9 ,
- Livia Puljak ORCID: orcid.org/0000-0002-8467-6061 10 ,
- Vinicius Tassoni Civile 11 ,
- Irena Zakarija-Grkovic 9 ,
- Tina Poklepovic Pericic 9 ,
- Alvaro Nagib Atallah 11 ,
- Santino Filoso 12 ,
- Nicola Luigi Bragazzi 13 &
- Milena Soriano Marcolino 1
On behalf of the International Network of Coronavirus Disease 2019 (InterNetCOVID-19)
BMC Infectious Diseases volume 21 , Article number: 525 ( 2021 ) Cite this article
15k Accesses
28 Citations
13 Altmetric
Metrics details
Navigating the rapidly growing body of scientific literature on the SARS-CoV-2 pandemic is challenging, and ongoing critical appraisal of this output is essential. We aimed to summarize and critically appraise systematic reviews of coronavirus disease (COVID-19) in humans that were available at the beginning of the pandemic.
Nine databases (Medline, EMBASE, Cochrane Library, CINAHL, Web of Sciences, PDQ-Evidence, WHO’s Global Research, LILACS, and Epistemonikos) were searched from December 1, 2019, to March 24, 2020. Systematic reviews analyzing primary studies of COVID-19 were included. Two authors independently undertook screening, selection, extraction (data on clinical symptoms, prevalence, pharmacological and non-pharmacological interventions, diagnostic test assessment, laboratory, and radiological findings), and quality assessment (AMSTAR 2). A meta-analysis was performed of the prevalence of clinical outcomes.
Eighteen systematic reviews were included; one was empty (did not identify any relevant study). Using AMSTAR 2, confidence in the results of all 18 reviews was rated as “critically low”. Identified symptoms of COVID-19 were (range values of point estimates): fever (82–95%), cough with or without sputum (58–72%), dyspnea (26–59%), myalgia or muscle fatigue (29–51%), sore throat (10–13%), headache (8–12%) and gastrointestinal complaints (5–9%). Severe symptoms were more common in men. Elevated C-reactive protein and lactate dehydrogenase, and slightly elevated aspartate and alanine aminotransferase, were commonly described. Thrombocytopenia and elevated levels of procalcitonin and cardiac troponin I were associated with severe disease. A frequent finding on chest imaging was uni- or bilateral multilobar ground-glass opacity. A single review investigated the impact of medication (chloroquine) but found no verifiable clinical data. All-cause mortality ranged from 0.3 to 13.9%.
Conclusions
In this overview of systematic reviews, we analyzed evidence from the first 18 systematic reviews that were published after the emergence of COVID-19. However, confidence in the results of all reviews was “critically low”. Thus, systematic reviews that were published early on in the pandemic were of questionable usefulness. Even during public health emergencies, studies and systematic reviews should adhere to established methodological standards.
Peer Review reports
The spread of the “Severe Acute Respiratory Coronavirus 2” (SARS-CoV-2), the causal agent of COVID-19, was characterized as a pandemic by the World Health Organization (WHO) in March 2020 and has triggered an international public health emergency [ 1 ]. The numbers of confirmed cases and deaths due to COVID-19 are rapidly escalating, counting in millions [ 2 ], causing massive economic strain, and escalating healthcare and public health expenses [ 3 , 4 ].
The research community has responded by publishing an impressive number of scientific reports related to COVID-19. The world was alerted to the new disease at the beginning of 2020 [ 1 ], and by mid-March 2020, more than 2000 articles had been published on COVID-19 in scholarly journals, with 25% of them containing original data [ 5 ]. The living map of COVID-19 evidence, curated by the Evidence for Policy and Practice Information and Co-ordinating Centre (EPPI-Centre), contained more than 40,000 records by February 2021 [ 6 ]. More than 100,000 records on PubMed were labeled as “SARS-CoV-2 literature, sequence, and clinical content” by February 2021 [ 7 ].
Due to publication speed, the research community has voiced concerns regarding the quality and reproducibility of evidence produced during the COVID-19 pandemic, warning of the potential damaging approach of “publish first, retract later” [ 8 ]. It appears that these concerns are not unfounded, as it has been reported that COVID-19 articles were overrepresented in the pool of retracted articles in 2020 [ 9 ]. These concerns about inadequate evidence are of major importance because they can lead to poor clinical practice and inappropriate policies [ 10 ].
Systematic reviews are a cornerstone of today’s evidence-informed decision-making. By synthesizing all relevant evidence regarding a particular topic, systematic reviews reflect the current scientific knowledge. Systematic reviews are considered to be at the highest level in the hierarchy of evidence and should be used to make informed decisions. However, with high numbers of systematic reviews of different scope and methodological quality being published, overviews of multiple systematic reviews that assess their methodological quality are essential [ 11 , 12 , 13 ]. An overview of systematic reviews helps identify and organize the literature and highlights areas of priority in decision-making.
In this overview of systematic reviews, we aimed to summarize and critically appraise systematic reviews of coronavirus disease (COVID-19) in humans that were available at the beginning of the pandemic.
Methodology
Research question.
This overview’s primary objective was to summarize and critically appraise systematic reviews that assessed any type of primary clinical data from patients infected with SARS-CoV-2. Our research question was purposefully broad because we wanted to analyze as many systematic reviews as possible that were available early following the COVID-19 outbreak.
Study design
We conducted an overview of systematic reviews. The idea for this overview originated in a protocol for a systematic review submitted to PROSPERO (CRD42020170623), which indicated a plan to conduct an overview.
Overviews of systematic reviews use explicit and systematic methods for searching and identifying multiple systematic reviews addressing related research questions in the same field to extract and analyze evidence across important outcomes. Overviews of systematic reviews are in principle similar to systematic reviews of interventions, but the unit of analysis is a systematic review [ 14 , 15 , 16 ].
We used the overview methodology instead of other evidence synthesis methods to allow us to collate and appraise multiple systematic reviews on this topic, and to extract and analyze their results across relevant topics [ 17 ]. The overview and meta-analysis of systematic reviews allowed us to investigate the methodological quality of included studies, summarize results, and identify specific areas of available or limited evidence, thereby strengthening the current understanding of this novel disease and guiding future research [ 13 ].
A reporting guideline for overviews of reviews is currently under development, i.e., Preferred Reporting Items for Overviews of Reviews (PRIOR) [ 18 ]. As the PRIOR checklist is still not published, this study was reported following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2009 statement [ 19 ]. The methodology used in this review was adapted from the Cochrane Handbook for Systematic Reviews of Interventions and also followed established methodological considerations for analyzing existing systematic reviews [ 14 ].
Approval of a research ethics committee was not necessary as the study analyzed only publicly available articles.
Eligibility criteria
Systematic reviews were included if they analyzed primary data from patients infected with SARS-CoV-2 as confirmed by RT-PCR or another pre-specified diagnostic technique. Eligible reviews covered all topics related to COVID-19 including, but not limited to, those that reported clinical symptoms, diagnostic methods, therapeutic interventions, laboratory findings, or radiological results. Both full manuscripts and abbreviated versions, such as letters, were eligible.
No restrictions were imposed on the design of the primary studies included within the systematic reviews, the last search date, whether the review included meta-analyses or language. Reviews related to SARS-CoV-2 and other coronaviruses were eligible, but from those reviews, we analyzed only data related to SARS-CoV-2.
No consensus definition exists for a systematic review [ 20 ], and debates continue about the defining characteristics of a systematic review [ 21 ]. Cochrane’s guidance for overviews of reviews recommends setting pre-established criteria for making decisions around inclusion [ 14 ]. That is supported by a recent scoping review about guidance for overviews of systematic reviews [ 22 ].
Thus, for this study, we defined a systematic review as a research report which searched for primary research studies on a specific topic using an explicit search strategy, had a detailed description of the methods with explicit inclusion criteria provided, and provided a summary of the included studies either in narrative or quantitative format (such as a meta-analysis). Cochrane and non-Cochrane systematic reviews were considered eligible for inclusion, with or without meta-analysis, and regardless of the study design, language restriction and methodology of the included primary studies. To be eligible for inclusion, reviews had to be clearly analyzing data related to SARS-CoV-2 (associated or not with other viruses). We excluded narrative reviews without those characteristics as these are less likely to be replicable and are more prone to bias.
Scoping reviews and rapid reviews were eligible for inclusion in this overview if they met our pre-defined inclusion criteria noted above. We included reviews that addressed SARS-CoV-2 and other coronaviruses if they reported separate data regarding SARS-CoV-2.
Information sources
Nine databases were searched for eligible records published between December 1, 2019, and March 24, 2020: Cochrane Database of Systematic Reviews via Cochrane Library, PubMed, EMBASE, CINAHL (Cumulative Index to Nursing and Allied Health Literature), Web of Sciences, LILACS (Latin American and Caribbean Health Sciences Literature), PDQ-Evidence, WHO’s Global Research on Coronavirus Disease (COVID-19), and Epistemonikos.
The comprehensive search strategy for each database is provided in Additional file 1 and was designed and conducted in collaboration with an information specialist. All retrieved records were primarily processed in EndNote, where duplicates were removed, and records were then imported into the Covidence platform [ 23 ]. In addition to database searches, we screened reference lists of reviews included after screening records retrieved via databases.
Study selection
All searches, screening of titles and abstracts, and record selection, were performed independently by two investigators using the Covidence platform [ 23 ]. Articles deemed potentially eligible were retrieved for full-text screening carried out independently by two investigators. Discrepancies at all stages were resolved by consensus. During the screening, records published in languages other than English were translated by a native/fluent speaker.
Data collection process
We custom designed a data extraction table for this study, which was piloted by two authors independently. Data extraction was performed independently by two authors. Conflicts were resolved by consensus or by consulting a third researcher.
We extracted the following data: article identification data (authors’ name and journal of publication), search period, number of databases searched, population or settings considered, main results and outcomes observed, and number of participants. From Web of Science (Clarivate Analytics, Philadelphia, PA, USA), we extracted journal rank (quartile) and Journal Impact Factor (JIF).
We categorized the following as primary outcomes: all-cause mortality, need for and length of mechanical ventilation, length of hospitalization (in days), admission to intensive care unit (yes/no), and length of stay in the intensive care unit.
The following outcomes were categorized as exploratory: diagnostic methods used for detection of the virus, male to female ratio, clinical symptoms, pharmacological and non-pharmacological interventions, laboratory findings (full blood count, liver enzymes, C-reactive protein, d-dimer, albumin, lipid profile, serum electrolytes, blood vitamin levels, glucose levels, and any other important biomarkers), and radiological findings (using radiography, computed tomography, magnetic resonance imaging or ultrasound).
We also collected data on reporting guidelines and requirements for the publication of systematic reviews and meta-analyses from journal websites where included reviews were published.
Quality assessment in individual reviews
Two researchers independently assessed the reviews’ quality using the “A MeaSurement Tool to Assess Systematic Reviews 2 (AMSTAR 2)”. We acknowledge that the AMSTAR 2 was created as “a critical appraisal tool for systematic reviews that include randomized or non-randomized studies of healthcare interventions, or both” [ 24 ]. However, since AMSTAR 2 was designed for systematic reviews of intervention trials, and we included additional types of systematic reviews, we adjusted some AMSTAR 2 ratings and reported these in Additional file 2 .
Adherence to each item was rated as follows: yes, partial yes, no, or not applicable (such as when a meta-analysis was not conducted). The overall confidence in the results of the review is rated as “critically low”, “low”, “moderate” or “high”, according to the AMSTAR 2 guidance based on seven critical domains, which are items 2, 4, 7, 9, 11, 13, 15 as defined by AMSTAR 2 authors [ 24 ]. We reported our adherence ratings for transparency of our decision with accompanying explanations, for each item, in each included review.
One of the included systematic reviews was conducted by some members of this author team [ 25 ]. This review was initially assessed independently by two authors who were not co-authors of that review to prevent the risk of bias in assessing this study.
Synthesis of results
For data synthesis, we prepared a table summarizing each systematic review. Graphs illustrating the mortality rate and clinical symptoms were created. We then prepared a narrative summary of the methods, findings, study strengths, and limitations.
For analysis of the prevalence of clinical outcomes, we extracted data on the number of events and the total number of patients to perform proportional meta-analysis using RStudio© software, with the “meta” package (version 4.9–6), using the “metaprop” function for reviews that did not perform a meta-analysis, excluding case studies because of the absence of variance. For reviews that did not perform a meta-analysis, we presented pooled results of proportions with their respective confidence intervals (95%) by the inverse variance method with a random-effects model, using the DerSimonian-Laird estimator for τ 2 . We adjusted data using Freeman-Tukey double arcosen transformation. Confidence intervals were calculated using the Clopper-Pearson method for individual studies. We created forest plots using the RStudio© software, with the “metafor” package (version 2.1–0) and “forest” function.
Managing overlapping systematic reviews
Some of the included systematic reviews that address the same or similar research questions may include the same primary studies in overviews. Including such overlapping reviews may introduce bias when outcome data from the same primary study are included in the analyses of an overview multiple times. Thus, in summaries of evidence, multiple-counting of the same outcome data will give data from some primary studies too much influence [ 14 ]. In this overview, we did not exclude overlapping systematic reviews because, according to Cochrane’s guidance, it may be appropriate to include all relevant reviews’ results if the purpose of the overview is to present and describe the current body of evidence on a topic [ 14 ]. To avoid any bias in summary estimates associated with overlapping reviews, we generated forest plots showing data from individual systematic reviews, but the results were not pooled because some primary studies were included in multiple reviews.
Our search retrieved 1063 publications, of which 175 were duplicates. Most publications were excluded after the title and abstract analysis ( n = 860). Among the 28 studies selected for full-text screening, 10 were excluded for the reasons described in Additional file 3 , and 18 were included in the final analysis (Fig. 1 ) [ 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 ]. Reference list screening did not retrieve any additional systematic reviews.
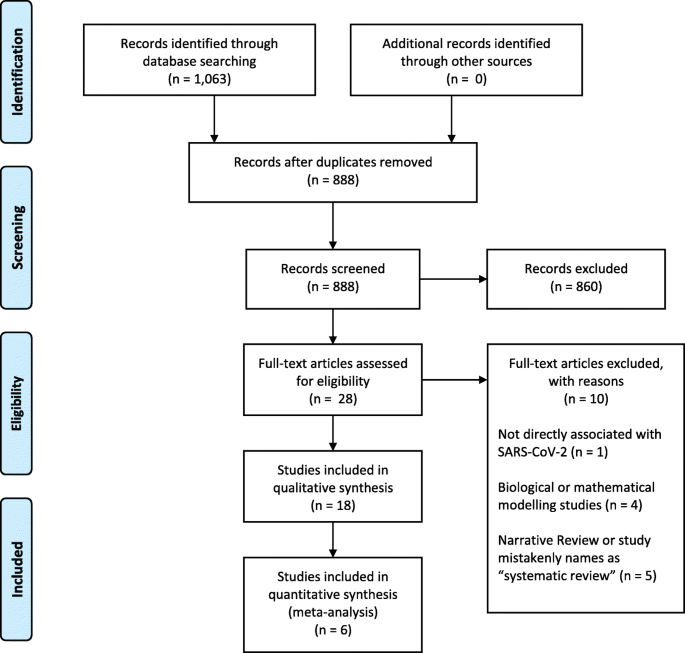
PRISMA flow diagram
Characteristics of included reviews
Summary features of 18 systematic reviews are presented in Table 1 . They were published in 14 different journals. Only four of these journals had specific requirements for systematic reviews (with or without meta-analysis): European Journal of Internal Medicine, Journal of Clinical Medicine, Ultrasound in Obstetrics and Gynecology, and Clinical Research in Cardiology . Two journals reported that they published only invited reviews ( Journal of Medical Virology and Clinica Chimica Acta ). Three systematic reviews in our study were published as letters; one was labeled as a scoping review and another as a rapid review (Table 2 ).
All reviews were published in English, in first quartile (Q1) journals, with JIF ranging from 1.692 to 6.062. One review was empty, meaning that its search did not identify any relevant studies; i.e., no primary studies were included [ 36 ]. The remaining 17 reviews included 269 unique studies; the majority ( N = 211; 78%) were included in only a single review included in our study (range: 1 to 12). Primary studies included in the reviews were published between December 2019 and March 18, 2020, and comprised case reports, case series, cohorts, and other observational studies. We found only one review that included randomized clinical trials [ 38 ]. In the included reviews, systematic literature searches were performed from 2019 (entire year) up to March 9, 2020. Ten systematic reviews included meta-analyses. The list of primary studies found in the included systematic reviews is shown in Additional file 4 , as well as the number of reviews in which each primary study was included.
Population and study designs
Most of the reviews analyzed data from patients with COVID-19 who developed pneumonia, acute respiratory distress syndrome (ARDS), or any other correlated complication. One review aimed to evaluate the effectiveness of using surgical masks on preventing transmission of the virus [ 36 ], one review was focused on pediatric patients [ 34 ], and one review investigated COVID-19 in pregnant women [ 37 ]. Most reviews assessed clinical symptoms, laboratory findings, or radiological results.
Systematic review findings
The summary of findings from individual reviews is shown in Table 2 . Overall, all-cause mortality ranged from 0.3 to 13.9% (Fig. 2 ).
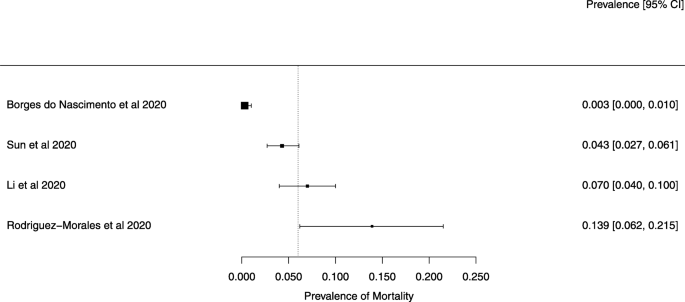
A meta-analysis of the prevalence of mortality
Clinical symptoms
Seven reviews described the main clinical manifestations of COVID-19 [ 26 , 28 , 29 , 34 , 35 , 39 , 41 ]. Three of them provided only a narrative discussion of symptoms [ 26 , 34 , 35 ]. In the reviews that performed a statistical analysis of the incidence of different clinical symptoms, symptoms in patients with COVID-19 were (range values of point estimates): fever (82–95%), cough with or without sputum (58–72%), dyspnea (26–59%), myalgia or muscle fatigue (29–51%), sore throat (10–13%), headache (8–12%), gastrointestinal disorders, such as diarrhea, nausea or vomiting (5.0–9.0%), and others (including, in one study only: dizziness 12.1%) (Figs. 3 , 4 , 5 , 6 , 7 , 8 and 9 ). Three reviews assessed cough with and without sputum together; only one review assessed sputum production itself (28.5%).
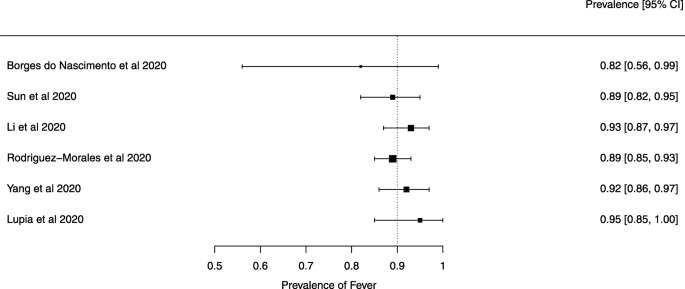
A meta-analysis of the prevalence of fever
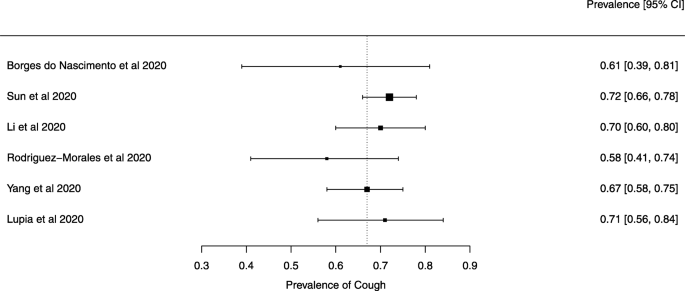
A meta-analysis of the prevalence of cough
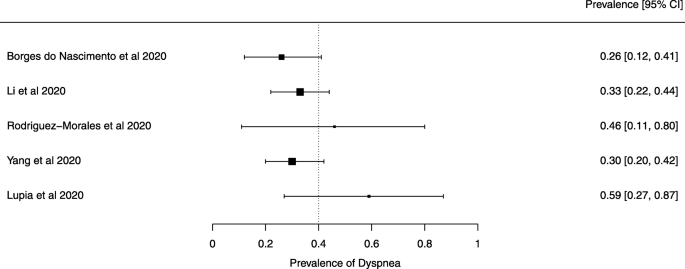
A meta-analysis of the prevalence of dyspnea
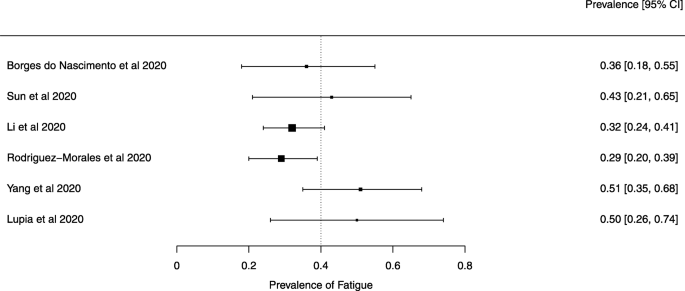
A meta-analysis of the prevalence of fatigue or myalgia
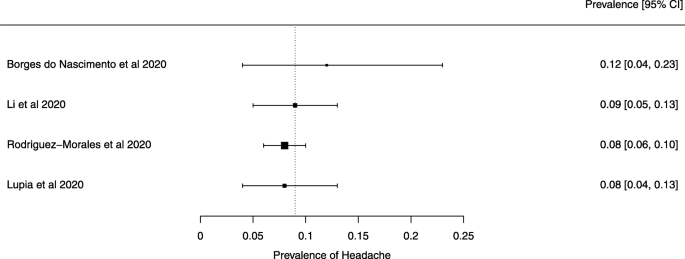
A meta-analysis of the prevalence of headache
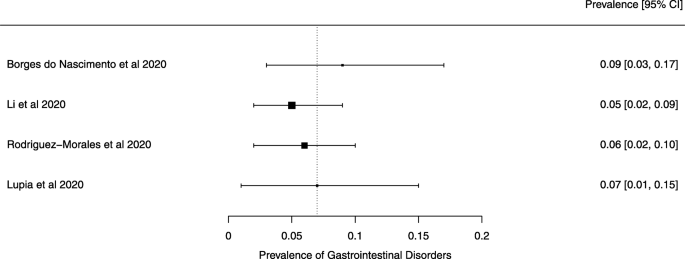
A meta-analysis of the prevalence of gastrointestinal disorders
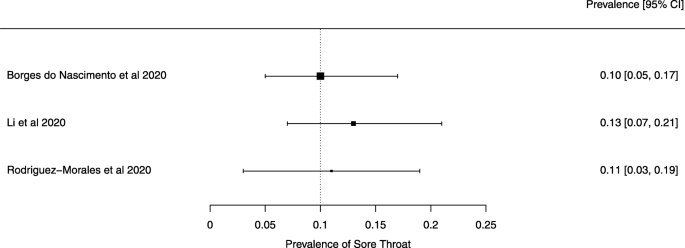
A meta-analysis of the prevalence of sore throat
Diagnostic aspects
Three reviews described methodologies, protocols, and tools used for establishing the diagnosis of COVID-19 [ 26 , 34 , 38 ]. The use of respiratory swabs (nasal or pharyngeal) or blood specimens to assess the presence of SARS-CoV-2 nucleic acid using RT-PCR assays was the most commonly used diagnostic method mentioned in the included studies. These diagnostic tests have been widely used, but their precise sensitivity and specificity remain unknown. One review included a Chinese study with clinical diagnosis with no confirmation of SARS-CoV-2 infection (patients were diagnosed with COVID-19 if they presented with at least two symptoms suggestive of COVID-19, together with laboratory and chest radiography abnormalities) [ 34 ].
Therapeutic possibilities
Pharmacological and non-pharmacological interventions (supportive therapies) used in treating patients with COVID-19 were reported in five reviews [ 25 , 27 , 34 , 35 , 38 ]. Antivirals used empirically for COVID-19 treatment were reported in seven reviews [ 25 , 27 , 34 , 35 , 37 , 38 , 41 ]; most commonly used were protease inhibitors (lopinavir, ritonavir, darunavir), nucleoside reverse transcriptase inhibitor (tenofovir), nucleotide analogs (remdesivir, galidesivir, ganciclovir), and neuraminidase inhibitors (oseltamivir). Umifenovir, a membrane fusion inhibitor, was investigated in two studies [ 25 , 35 ]. Possible supportive interventions analyzed were different types of oxygen supplementation and breathing support (invasive or non-invasive ventilation) [ 25 ]. The use of antibiotics, both empirically and to treat secondary pneumonia, was reported in six studies [ 25 , 26 , 27 , 34 , 35 , 38 ]. One review specifically assessed evidence on the efficacy and safety of the anti-malaria drug chloroquine [ 27 ]. It identified 23 ongoing trials investigating the potential of chloroquine as a therapeutic option for COVID-19, but no verifiable clinical outcomes data. The use of mesenchymal stem cells, antifungals, and glucocorticoids were described in four reviews [ 25 , 34 , 35 , 38 ].
Laboratory and radiological findings
Of the 18 reviews included in this overview, eight analyzed laboratory parameters in patients with COVID-19 [ 25 , 29 , 30 , 32 , 33 , 34 , 35 , 39 ]; elevated C-reactive protein levels, associated with lymphocytopenia, elevated lactate dehydrogenase, as well as slightly elevated aspartate and alanine aminotransferase (AST, ALT) were commonly described in those eight reviews. Lippi et al. assessed cardiac troponin I (cTnI) [ 25 ], procalcitonin [ 32 ], and platelet count [ 33 ] in COVID-19 patients. Elevated levels of procalcitonin [ 32 ] and cTnI [ 30 ] were more likely to be associated with a severe disease course (requiring intensive care unit admission and intubation). Furthermore, thrombocytopenia was frequently observed in patients with complicated COVID-19 infections [ 33 ].
Chest imaging (chest radiography and/or computed tomography) features were assessed in six reviews, all of which described a frequent pattern of local or bilateral multilobar ground-glass opacity [ 25 , 34 , 35 , 39 , 40 , 41 ]. Those six reviews showed that septal thickening, bronchiectasis, pleural and cardiac effusions, halo signs, and pneumothorax were observed in patients suffering from COVID-19.
Quality of evidence in individual systematic reviews
Table 3 shows the detailed results of the quality assessment of 18 systematic reviews, including the assessment of individual items and summary assessment. A detailed explanation for each decision in each review is available in Additional file 5 .
Using AMSTAR 2 criteria, confidence in the results of all 18 reviews was rated as “critically low” (Table 3 ). Common methodological drawbacks were: omission of prospective protocol submission or publication; use of inappropriate search strategy: lack of independent and dual literature screening and data-extraction (or methodology unclear); absence of an explanation for heterogeneity among the studies included; lack of reasons for study exclusion (or rationale unclear).
Risk of bias assessment, based on a reported methodological tool, and quality of evidence appraisal, in line with the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) method, were reported only in one review [ 25 ]. Five reviews presented a table summarizing bias, using various risk of bias tools [ 25 , 29 , 39 , 40 , 41 ]. One review analyzed “study quality” [ 37 ]. One review mentioned the risk of bias assessment in the methodology but did not provide any related analysis [ 28 ].
This overview of systematic reviews analyzed the first 18 systematic reviews published after the onset of the COVID-19 pandemic, up to March 24, 2020, with primary studies involving more than 60,000 patients. Using AMSTAR-2, we judged that our confidence in all those reviews was “critically low”. Ten reviews included meta-analyses. The reviews presented data on clinical manifestations, laboratory and radiological findings, and interventions. We found no systematic reviews on the utility of diagnostic tests.
Symptoms were reported in seven reviews; most of the patients had a fever, cough, dyspnea, myalgia or muscle fatigue, and gastrointestinal disorders such as diarrhea, nausea, or vomiting. Olfactory dysfunction (anosmia or dysosmia) has been described in patients infected with COVID-19 [ 43 ]; however, this was not reported in any of the reviews included in this overview. During the SARS outbreak in 2002, there were reports of impairment of the sense of smell associated with the disease [ 44 , 45 ].
The reported mortality rates ranged from 0.3 to 14% in the included reviews. Mortality estimates are influenced by the transmissibility rate (basic reproduction number), availability of diagnostic tools, notification policies, asymptomatic presentations of the disease, resources for disease prevention and control, and treatment facilities; variability in the mortality rate fits the pattern of emerging infectious diseases [ 46 ]. Furthermore, the reported cases did not consider asymptomatic cases, mild cases where individuals have not sought medical treatment, and the fact that many countries had limited access to diagnostic tests or have implemented testing policies later than the others. Considering the lack of reviews assessing diagnostic testing (sensitivity, specificity, and predictive values of RT-PCT or immunoglobulin tests), and the preponderance of studies that assessed only symptomatic individuals, considerable imprecision around the calculated mortality rates existed in the early stage of the COVID-19 pandemic.
Few reviews included treatment data. Those reviews described studies considered to be at a very low level of evidence: usually small, retrospective studies with very heterogeneous populations. Seven reviews analyzed laboratory parameters; those reviews could have been useful for clinicians who attend patients suspected of COVID-19 in emergency services worldwide, such as assessing which patients need to be reassessed more frequently.
All systematic reviews scored poorly on the AMSTAR 2 critical appraisal tool for systematic reviews. Most of the original studies included in the reviews were case series and case reports, impacting the quality of evidence. Such evidence has major implications for clinical practice and the use of these reviews in evidence-based practice and policy. Clinicians, patients, and policymakers can only have the highest confidence in systematic review findings if high-quality systematic review methodologies are employed. The urgent need for information during a pandemic does not justify poor quality reporting.
We acknowledge that there are numerous challenges associated with analyzing COVID-19 data during a pandemic [ 47 ]. High-quality evidence syntheses are needed for decision-making, but each type of evidence syntheses is associated with its inherent challenges.
The creation of classic systematic reviews requires considerable time and effort; with massive research output, they quickly become outdated, and preparing updated versions also requires considerable time. A recent study showed that updates of non-Cochrane systematic reviews are published a median of 5 years after the publication of the previous version [ 48 ].
Authors may register a review and then abandon it [ 49 ], but the existence of a public record that is not updated may lead other authors to believe that the review is still ongoing. A quarter of Cochrane review protocols remains unpublished as completed systematic reviews 8 years after protocol publication [ 50 ].
Rapid reviews can be used to summarize the evidence, but they involve methodological sacrifices and simplifications to produce information promptly, with inconsistent methodological approaches [ 51 ]. However, rapid reviews are justified in times of public health emergencies, and even Cochrane has resorted to publishing rapid reviews in response to the COVID-19 crisis [ 52 ]. Rapid reviews were eligible for inclusion in this overview, but only one of the 18 reviews included in this study was labeled as a rapid review.
Ideally, COVID-19 evidence would be continually summarized in a series of high-quality living systematic reviews, types of evidence synthesis defined as “ a systematic review which is continually updated, incorporating relevant new evidence as it becomes available ” [ 53 ]. However, conducting living systematic reviews requires considerable resources, calling into question the sustainability of such evidence synthesis over long periods [ 54 ].
Research reports about COVID-19 will contribute to research waste if they are poorly designed, poorly reported, or simply not necessary. In principle, systematic reviews should help reduce research waste as they usually provide recommendations for further research that is needed or may advise that sufficient evidence exists on a particular topic [ 55 ]. However, systematic reviews can also contribute to growing research waste when they are not needed, or poorly conducted and reported. Our present study clearly shows that most of the systematic reviews that were published early on in the COVID-19 pandemic could be categorized as research waste, as our confidence in their results is critically low.
Our study has some limitations. One is that for AMSTAR 2 assessment we relied on information available in publications; we did not attempt to contact study authors for clarifications or additional data. In three reviews, the methodological quality appraisal was challenging because they were published as letters, or labeled as rapid communications. As a result, various details about their review process were not included, leading to AMSTAR 2 questions being answered as “not reported”, resulting in low confidence scores. Full manuscripts might have provided additional information that could have led to higher confidence in the results. In other words, low scores could reflect incomplete reporting, not necessarily low-quality review methods. To make their review available more rapidly and more concisely, the authors may have omitted methodological details. A general issue during a crisis is that speed and completeness must be balanced. However, maintaining high standards requires proper resourcing and commitment to ensure that the users of systematic reviews can have high confidence in the results.
Furthermore, we used adjusted AMSTAR 2 scoring, as the tool was designed for critical appraisal of reviews of interventions. Some reviews may have received lower scores than actually warranted in spite of these adjustments.
Another limitation of our study may be the inclusion of multiple overlapping reviews, as some included reviews included the same primary studies. According to the Cochrane Handbook, including overlapping reviews may be appropriate when the review’s aim is “ to present and describe the current body of systematic review evidence on a topic ” [ 12 ], which was our aim. To avoid bias with summarizing evidence from overlapping reviews, we presented the forest plots without summary estimates. The forest plots serve to inform readers about the effect sizes for outcomes that were reported in each review.
Several authors from this study have contributed to one of the reviews identified [ 25 ]. To reduce the risk of any bias, two authors who did not co-author the review in question initially assessed its quality and limitations.
Finally, we note that the systematic reviews included in our overview may have had issues that our analysis did not identify because we did not analyze their primary studies to verify the accuracy of the data and information they presented. We give two examples to substantiate this possibility. Lovato et al. wrote a commentary on the review of Sun et al. [ 41 ], in which they criticized the authors’ conclusion that sore throat is rare in COVID-19 patients [ 56 ]. Lovato et al. highlighted that multiple studies included in Sun et al. did not accurately describe participants’ clinical presentations, warning that only three studies clearly reported data on sore throat [ 56 ].
In another example, Leung [ 57 ] warned about the review of Li, L.Q. et al. [ 29 ]: “ it is possible that this statistic was computed using overlapped samples, therefore some patients were double counted ”. Li et al. responded to Leung that it is uncertain whether the data overlapped, as they used data from published articles and did not have access to the original data; they also reported that they requested original data and that they plan to re-do their analyses once they receive them; they also urged readers to treat the data with caution [ 58 ]. This points to the evolving nature of evidence during a crisis.
Our study’s strength is that this overview adds to the current knowledge by providing a comprehensive summary of all the evidence synthesis about COVID-19 available early after the onset of the pandemic. This overview followed strict methodological criteria, including a comprehensive and sensitive search strategy and a standard tool for methodological appraisal of systematic reviews.
In conclusion, in this overview of systematic reviews, we analyzed evidence from the first 18 systematic reviews that were published after the emergence of COVID-19. However, confidence in the results of all the reviews was “critically low”. Thus, systematic reviews that were published early on in the pandemic could be categorized as research waste. Even during public health emergencies, studies and systematic reviews should adhere to established methodological standards to provide patients, clinicians, and decision-makers trustworthy evidence.
Availability of data and materials
All data collected and analyzed within this study are available from the corresponding author on reasonable request.
World Health Organization. Timeline - COVID-19: Available at: https://www.who.int/news/item/29-06-2020-covidtimeline . Accessed 1 June 2021.
COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). Available at: https://coronavirus.jhu.edu/map.html . Accessed 1 June 2021.
Anzai A, Kobayashi T, Linton NM, Kinoshita R, Hayashi K, Suzuki A, et al. Assessing the Impact of Reduced Travel on Exportation Dynamics of Novel Coronavirus Infection (COVID-19). J Clin Med. 2020;9(2):601.
Chinazzi M, Davis JT, Ajelli M, Gioannini C, Litvinova M, Merler S, et al. The effect of travel restrictions on the spread of the 2019 novel coronavirus (COVID-19) outbreak. Science. 2020;368(6489):395–400. https://doi.org/10.1126/science.aba9757 .
Article CAS PubMed PubMed Central Google Scholar
Fidahic M, Nujic D, Runjic R, Civljak M, Markotic F, Lovric Makaric Z, et al. Research methodology and characteristics of journal articles with original data, preprint articles and registered clinical trial protocols about COVID-19. BMC Med Res Methodol. 2020;20(1):161. https://doi.org/10.1186/s12874-020-01047-2 .
EPPI Centre . COVID-19: a living systematic map of the evidence. Available at: http://eppi.ioe.ac.uk/cms/Projects/DepartmentofHealthandSocialCare/Publishedreviews/COVID-19Livingsystematicmapoftheevidence/tabid/3765/Default.aspx . Accessed 1 June 2021.
NCBI SARS-CoV-2 Resources. Available at: https://www.ncbi.nlm.nih.gov/sars-cov-2/ . Accessed 1 June 2021.
Gustot T. Quality and reproducibility during the COVID-19 pandemic. JHEP Rep. 2020;2(4):100141. https://doi.org/10.1016/j.jhepr.2020.100141 .
Article PubMed PubMed Central Google Scholar
Kodvanj, I., et al., Publishing of COVID-19 Preprints in Peer-reviewed Journals, Preprinting Trends, Public Discussion and Quality Issues. Preprint article. bioRxiv 2020.11.23.394577; doi: https://doi.org/10.1101/2020.11.23.394577 .
Dobler CC. Poor quality research and clinical practice during COVID-19. Breathe (Sheff). 2020;16(2):200112. https://doi.org/10.1183/20734735.0112-2020 .
Article Google Scholar
Bastian H, Glasziou P, Chalmers I. Seventy-five trials and eleven systematic reviews a day: how will we ever keep up? PLoS Med. 2010;7(9):e1000326. https://doi.org/10.1371/journal.pmed.1000326 .
Lunny C, Brennan SE, McDonald S, McKenzie JE. Toward a comprehensive evidence map of overview of systematic review methods: paper 1-purpose, eligibility, search and data extraction. Syst Rev. 2017;6(1):231. https://doi.org/10.1186/s13643-017-0617-1 .
Pollock M, Fernandes RM, Becker LA, Pieper D, Hartling L. Chapter V: Overviews of Reviews. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane. 2020. Available from www.training.cochrane.org/handbook .
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions version 6.1 (updated September 2020). Cochrane. 2020; Available from www.training.cochrane.org/handbook .
Pollock M, Fernandes RM, Newton AS, Scott SD, Hartling L. The impact of different inclusion decisions on the comprehensiveness and complexity of overviews of reviews of healthcare interventions. Syst Rev. 2019;8(1):18. https://doi.org/10.1186/s13643-018-0914-3 .
Pollock M, Fernandes RM, Newton AS, Scott SD, Hartling L. A decision tool to help researchers make decisions about including systematic reviews in overviews of reviews of healthcare interventions. Syst Rev. 2019;8(1):29. https://doi.org/10.1186/s13643-018-0768-8 .
Hunt H, Pollock A, Campbell P, Estcourt L, Brunton G. An introduction to overviews of reviews: planning a relevant research question and objective for an overview. Syst Rev. 2018;7(1):39. https://doi.org/10.1186/s13643-018-0695-8 .
Pollock M, Fernandes RM, Pieper D, Tricco AC, Gates M, Gates A, et al. Preferred reporting items for overviews of reviews (PRIOR): a protocol for development of a reporting guideline for overviews of reviews of healthcare interventions. Syst Rev. 2019;8(1):335. https://doi.org/10.1186/s13643-019-1252-9 .
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Open Med. 2009;3(3):e123–30.
Krnic Martinic M, Pieper D, Glatt A, Puljak L. Definition of a systematic review used in overviews of systematic reviews, meta-epidemiological studies and textbooks. BMC Med Res Methodol. 2019;19(1):203. https://doi.org/10.1186/s12874-019-0855-0 .
Puljak L. If there is only one author or only one database was searched, a study should not be called a systematic review. J Clin Epidemiol. 2017;91:4–5. https://doi.org/10.1016/j.jclinepi.2017.08.002 .
Article PubMed Google Scholar
Gates M, Gates A, Guitard S, Pollock M, Hartling L. Guidance for overviews of reviews continues to accumulate, but important challenges remain: a scoping review. Syst Rev. 2020;9(1):254. https://doi.org/10.1186/s13643-020-01509-0 .
Covidence - systematic review software. Available at: https://www.covidence.org/ . Accessed 1 June 2021.
Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008.
Borges do Nascimento IJ, et al. Novel Coronavirus Infection (COVID-19) in Humans: A Scoping Review and Meta-Analysis. J Clin Med. 2020;9(4):941.
Article PubMed Central Google Scholar
Adhikari SP, Meng S, Wu YJ, Mao YP, Ye RX, Wang QZ, et al. Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID-19) during the early outbreak period: a scoping review. Infect Dis Poverty. 2020;9(1):29. https://doi.org/10.1186/s40249-020-00646-x .
Cortegiani A, Ingoglia G, Ippolito M, Giarratano A, Einav S. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J Crit Care. 2020;57:279–83. https://doi.org/10.1016/j.jcrc.2020.03.005 .
Li B, Yang J, Zhao F, Zhi L, Wang X, Liu L, et al. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020;109(5):531–8. https://doi.org/10.1007/s00392-020-01626-9 .
Article CAS PubMed Google Scholar
Li LQ, Huang T, Wang YQ, Wang ZP, Liang Y, Huang TB, et al. COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol. 2020;92(6):577–83. https://doi.org/10.1002/jmv.25757 .
Lippi G, Lavie CJ, Sanchis-Gomar F. Cardiac troponin I in patients with coronavirus disease 2019 (COVID-19): evidence from a meta-analysis. Prog Cardiovasc Dis. 2020;63(3):390–1. https://doi.org/10.1016/j.pcad.2020.03.001 .
Lippi G, Henry BM. Active smoking is not associated with severity of coronavirus disease 2019 (COVID-19). Eur J Intern Med. 2020;75:107–8. https://doi.org/10.1016/j.ejim.2020.03.014 .
Lippi G, Plebani M. Procalcitonin in patients with severe coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chim Acta. 2020;505:190–1. https://doi.org/10.1016/j.cca.2020.03.004 .
Lippi G, Plebani M, Henry BM. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a meta-analysis. Clin Chim Acta. 2020;506:145–8. https://doi.org/10.1016/j.cca.2020.03.022 .
Ludvigsson JF. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020;109(6):1088–95. https://doi.org/10.1111/apa.15270 .
Lupia T, Scabini S, Mornese Pinna S, di Perri G, de Rosa FG, Corcione S. 2019 novel coronavirus (2019-nCoV) outbreak: a new challenge. J Glob Antimicrob Resist. 2020;21:22–7. https://doi.org/10.1016/j.jgar.2020.02.021 .
Marasinghe, K.M., A systematic review investigating the effectiveness of face mask use in limiting the spread of COVID-19 among medically not diagnosed individuals: shedding light on current recommendations provided to individuals not medically diagnosed with COVID-19. Research Square. Preprint article. doi : https://doi.org/10.21203/rs.3.rs-16701/v1 . 2020 .
Mullins E, Evans D, Viner RM, O’Brien P, Morris E. Coronavirus in pregnancy and delivery: rapid review. Ultrasound Obstet Gynecol. 2020;55(5):586–92. https://doi.org/10.1002/uog.22014 .
Pang J, Wang MX, Ang IYH, Tan SHX, Lewis RF, Chen JIP, et al. Potential Rapid Diagnostics, Vaccine and Therapeutics for 2019 Novel coronavirus (2019-nCoV): a systematic review. J Clin Med. 2020;9(3):623.
Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, Villamizar-Peña R, Holguin-Rivera Y, Escalera-Antezana JP, et al. Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Travel Med Infect Dis. 2020;34:101623. https://doi.org/10.1016/j.tmaid.2020.101623 .
Salehi S, Abedi A, Balakrishnan S, Gholamrezanezhad A. Coronavirus disease 2019 (COVID-19): a systematic review of imaging findings in 919 patients. AJR Am J Roentgenol. 2020;215(1):87–93. https://doi.org/10.2214/AJR.20.23034 .
Sun P, Qie S, Liu Z, Ren J, Li K, Xi J. Clinical characteristics of hospitalized patients with SARS-CoV-2 infection: a single arm meta-analysis. J Med Virol. 2020;92(6):612–7. https://doi.org/10.1002/jmv.25735 .
Yang J, Zheng Y, Gou X, Pu K, Chen Z, Guo Q, et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–5. https://doi.org/10.1016/j.ijid.2020.03.017 .
Bassetti M, Vena A, Giacobbe DR. The novel Chinese coronavirus (2019-nCoV) infections: challenges for fighting the storm. Eur J Clin Investig. 2020;50(3):e13209. https://doi.org/10.1111/eci.13209 .
Article CAS Google Scholar
Hwang CS. Olfactory neuropathy in severe acute respiratory syndrome: report of a case. Acta Neurol Taiwanica. 2006;15(1):26–8.
Google Scholar
Suzuki M, Saito K, Min WP, Vladau C, Toida K, Itoh H, et al. Identification of viruses in patients with postviral olfactory dysfunction. Laryngoscope. 2007;117(2):272–7. https://doi.org/10.1097/01.mlg.0000249922.37381.1e .
Rajgor DD, Lee MH, Archuleta S, Bagdasarian N, Quek SC. The many estimates of the COVID-19 case fatality rate. Lancet Infect Dis. 2020;20(7):776–7. https://doi.org/10.1016/S1473-3099(20)30244-9 .
Wolkewitz M, Puljak L. Methodological challenges of analysing COVID-19 data during the pandemic. BMC Med Res Methodol. 2020;20(1):81. https://doi.org/10.1186/s12874-020-00972-6 .
Rombey T, Lochner V, Puljak L, Könsgen N, Mathes T, Pieper D. Epidemiology and reporting characteristics of non-Cochrane updates of systematic reviews: a cross-sectional study. Res Synth Methods. 2020;11(3):471–83. https://doi.org/10.1002/jrsm.1409 .
Runjic E, Rombey T, Pieper D, Puljak L. Half of systematic reviews about pain registered in PROSPERO were not published and the majority had inaccurate status. J Clin Epidemiol. 2019;116:114–21. https://doi.org/10.1016/j.jclinepi.2019.08.010 .
Runjic E, Behmen D, Pieper D, Mathes T, Tricco AC, Moher D, et al. Following Cochrane review protocols to completion 10 years later: a retrospective cohort study and author survey. J Clin Epidemiol. 2019;111:41–8. https://doi.org/10.1016/j.jclinepi.2019.03.006 .
Tricco AC, Antony J, Zarin W, Strifler L, Ghassemi M, Ivory J, et al. A scoping review of rapid review methods. BMC Med. 2015;13(1):224. https://doi.org/10.1186/s12916-015-0465-6 .
COVID-19 Rapid Reviews: Cochrane’s response so far. Available at: https://training.cochrane.org/resource/covid-19-rapid-reviews-cochrane-response-so-far . Accessed 1 June 2021.
Cochrane. Living systematic reviews. Available at: https://community.cochrane.org/review-production/production-resources/living-systematic-reviews . Accessed 1 June 2021.
Millard T, Synnot A, Elliott J, Green S, McDonald S, Turner T. Feasibility and acceptability of living systematic reviews: results from a mixed-methods evaluation. Syst Rev. 2019;8(1):325. https://doi.org/10.1186/s13643-019-1248-5 .
Babic A, Poklepovic Pericic T, Pieper D, Puljak L. How to decide whether a systematic review is stable and not in need of updating: analysis of Cochrane reviews. Res Synth Methods. 2020;11(6):884–90. https://doi.org/10.1002/jrsm.1451 .
Lovato A, Rossettini G, de Filippis C. Sore throat in COVID-19: comment on “clinical characteristics of hospitalized patients with SARS-CoV-2 infection: a single arm meta-analysis”. J Med Virol. 2020;92(7):714–5. https://doi.org/10.1002/jmv.25815 .
Leung C. Comment on Li et al: COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol. 2020;92(9):1431–2. https://doi.org/10.1002/jmv.25912 .
Li LQ, Huang T, Wang YQ, Wang ZP, Liang Y, Huang TB, et al. Response to Char’s comment: comment on Li et al: COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol. 2020;92(9):1433. https://doi.org/10.1002/jmv.25924 .
Download references
Acknowledgments
We thank Catherine Henderson DPhil from Swanscoe Communications for pro bono medical writing and editing support. We acknowledge support from the Covidence Team, specifically Anneliese Arno. We thank the whole International Network of Coronavirus Disease 2019 (InterNetCOVID-19) for their commitment and involvement. Members of the InterNetCOVID-19 are listed in Additional file 6 . We thank Pavel Cerny and Roger Crosthwaite for guiding the team supervisor (IJBN) on human resources management.
This research received no external funding.
Author information
Authors and affiliations.
University Hospital and School of Medicine, Universidade Federal de Minas Gerais, Belo Horizonte, Minas Gerais, Brazil
Israel Júnior Borges do Nascimento & Milena Soriano Marcolino
Medical College of Wisconsin, Milwaukee, WI, USA
Israel Júnior Borges do Nascimento
Helene Fuld Health Trust National Institute for Evidence-based Practice in Nursing and Healthcare, College of Nursing, The Ohio State University, Columbus, OH, USA
Dónal P. O’Mathúna
School of Nursing, Psychotherapy and Community Health, Dublin City University, Dublin, Ireland
Department of Anesthesiology, Intensive Care and Pain Medicine, University of Münster, Münster, Germany
Thilo Caspar von Groote
Department of Sport and Health Science, Technische Universität München, Munich, Germany
Hebatullah Mohamed Abdulazeem
School of Health Sciences, Faculty of Health and Medicine, The University of Newcastle, Callaghan, Australia
Ishanka Weerasekara
Department of Physiotherapy, Faculty of Allied Health Sciences, University of Peradeniya, Peradeniya, Sri Lanka
Cochrane Croatia, University of Split, School of Medicine, Split, Croatia
Ana Marusic, Irena Zakarija-Grkovic & Tina Poklepovic Pericic
Center for Evidence-Based Medicine and Health Care, Catholic University of Croatia, Ilica 242, 10000, Zagreb, Croatia
Livia Puljak
Cochrane Brazil, Evidence-Based Health Program, Universidade Federal de São Paulo, São Paulo, Brazil
Vinicius Tassoni Civile & Alvaro Nagib Atallah
Yorkville University, Fredericton, New Brunswick, Canada
Santino Filoso
Laboratory for Industrial and Applied Mathematics (LIAM), Department of Mathematics and Statistics, York University, Toronto, Ontario, Canada
Nicola Luigi Bragazzi
You can also search for this author in PubMed Google Scholar
Contributions
IJBN conceived the research idea and worked as a project coordinator. DPOM, TCVG, HMA, IW, AM, LP, VTC, IZG, TPP, ANA, SF, NLB and MSM were involved in data curation, formal analysis, investigation, methodology, and initial draft writing. All authors revised the manuscript critically for the content. The author(s) read and approved the final manuscript.
Corresponding author
Correspondence to Livia Puljak .
Ethics declarations
Ethics approval and consent to participate.
Not required as data was based on published studies.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: appendix 1..
Search strategies used in the study.
Additional file 2: Appendix 2.
Adjusted scoring of AMSTAR 2 used in this study for systematic reviews of studies that did not analyze interventions.
Additional file 3: Appendix 3.
List of excluded studies, with reasons.
Additional file 4: Appendix 4.
Table of overlapping studies, containing the list of primary studies included, their visual overlap in individual systematic reviews, and the number in how many reviews each primary study was included.
Additional file 5: Appendix 5.
A detailed explanation of AMSTAR scoring for each item in each review.
Additional file 6: Appendix 6.
List of members and affiliates of International Network of Coronavirus Disease 2019 (InterNetCOVID-19).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Borges do Nascimento, I.J., O’Mathúna, D.P., von Groote, T.C. et al. Coronavirus disease (COVID-19) pandemic: an overview of systematic reviews. BMC Infect Dis 21 , 525 (2021). https://doi.org/10.1186/s12879-021-06214-4
Download citation
Received : 12 April 2020
Accepted : 19 May 2021
Published : 04 June 2021
DOI : https://doi.org/10.1186/s12879-021-06214-4

Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Coronavirus
- Evidence-based medicine
- Infectious diseases
BMC Infectious Diseases
ISSN: 1471-2334
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 14 December 2021
COVID-19 in 2022: controlling the pandemic is within our grasp
- Maria D. Van Kerkhove ORCID: orcid.org/0000-0002-6135-0018 1
Nature Medicine volume 27 , page 2070 ( 2021 ) Cite this article
40k Accesses
18 Citations
217 Altmetric
Metrics details
- Respiratory tract diseases
- Scientific community
Vaccine inequity, inconsistent public health measures and new variants such as Omicron are prolonging the COVID-19 pandemic, but controlling the virus remains possible.
As we enter the third year of the COVID-19 pandemic, public health leaders must take stock of where we are and how we can end the crippling hold that the SARS-CoV-2 virus has over us all. All pandemics end. The COVID-19 pandemic will end, but it is not over yet. Already, we have endured two years of missed opportunities, missed education, missed connections with family and loved ones. Without action, 2022 could be the same. But it doesn’t have to be.

The virus will continue to affect our lives and livelihoods unless the global community collectively addresses inequitable access to vaccines, therapeutic agents and diagnostics, as well as the fact that we are giving the SARS-CoV-2 virus the room it needs to thrive through uneven and inconsistent national policies to reduce transmission, some of which are undermined by division and politicization. At the same time, governments must invest in preparedness, prevention and in science.
One of the greatest scientific achievements of the pandemic has been the speed of the development of several safe and effective COVID-19 vaccines. Robust data continue to show that COVID-19 vaccines are very effective at preventing people from getting seriously ill and dying. This protection seems to be maintained against the more transmissible Delta variant and over time .
However, we continue to see persistent inequities in access to COVID-19 vaccines. Of the more than 7 billion doses administered so far, less than 3% have been in countries on the African continent. The World Health Organization (WHO) has recommended that those at higher risk of severe disease and health workers be prioritised for COVID-19 vaccination in all countries. The number of doses administered by the end of September 2021 was enough to have covered 40% of the population in all countries. The failure to provide access of sufficient doses of vaccines to low- and middle-income countries is not only unethical, but it is epidemiologically and economically unwise, and is prolonging the pandemic.
Unfortunately, vaccines alone will not end this pandemic, in part because of more transmissible new variants and also because vaccines are primarily designed to protect against severe disease and death. The more the virus is allowed to circulate, the more opportunity the virus has to evolve. Throughout the course of the pandemic, SARS-CoV-2 has shown its ability to become better adapted to the human host, with variants Alpha and Delta demonstrating enhanced transmissibility. One of the biggest unknowns in 2022 will be how this evolution continues. Delta continues to evolve, and the Omicron variant has shown that the virus will continue to adapt, and such variants may be more transmissible, cause more or less severe disease, and/or develop properties of immune escape .
In 2022, epidemiological and genomic surveillance efforts should be expanded in all countries to detect SARS-CoV-2 variants and ensure that robust testing systems are linked to public health action. As we track the evolution of the virus, the WHO and partners will continue to closely assess and monitor the effect of virus evolution on public health and medical countermeasures, including diagnostics, therapeutic agents and COVID-19 vaccines .
Since the beginning of the pandemic, the WHO has recommended comprehensive measures to reduce SARS-CoV-2 transmission. Current vaccines by themselves are insufficient to stem transmission, and so increases in cases should be expected whenever public health and social measures are lifted, irrespective of vaccination coverage. In 2022, much of the world will need to continue with effective measures as we bring transmission under control. This includes, for example, wearing well-fitting masks, hand hygiene, physical distancing, improving ventilation of indoor spaces, avoiding crowded spaces and being supported to stay home if unwell.
It is essential to continue to improve national and sub-national public health infrastructure to better target and tailor local responses. Although the seasonal patterns of influenza and respiratory syncytial virus have been disrupted owing to the interventions for COVID-19, they have not disappeared from circulation, and governments should prepare for them to circulate again.
Preparedness for the next epidemic or pandemic pathogen does not begin when the current emergency is over. It begins now, with investing in integrated respiratory disease surveillance, a well-protected work force, early clinical care, access to health care, better personal protective equipment, further research and development for diagnostics, therapeutics and vaccines and addressing long-standing inequalities. This will not only help to control COVID-19, but also ensure that we are in a better position to confront the next outbreak.
In 2022, with increasing population level immunity, there will be substantial reductions in the number of people experiencing severe disease and death. This will alleviate some of the strain COVID-19 has exercised on even the most robust health-care systems. However, it is likely that there will be continued surges in transmission among unprotected individuals and strain on health-care systems in areas where vaccine coverage is low. The risk of more transmissible variants or variants with immune escape properties means that governments and communities must continue efforts to reduce transmission and protect the vulnerable, while slowly and carefully reopening our societies. Controlling this virus was always in our control, it remains in our control.
Author information
Authors and affiliations.
COVID-19 Health Operations and Technical Lead, Emerging Diseases and Zoonoses Unit Head, Health Emergencies Programme, World Health Organization, Geneva, Switzerland
Maria D. Van Kerkhove
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Maria D. Van Kerkhove .
Ethics declarations
Competing interests.
The author declares no competing interests.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Van Kerkhove, M.D. COVID-19 in 2022: controlling the pandemic is within our grasp. Nat Med 27 , 2070 (2021). https://doi.org/10.1038/s41591-021-01616-y
Download citation
Published : 14 December 2021
Issue Date : December 2021
DOI : https://doi.org/10.1038/s41591-021-01616-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Clinical courses of 24,563 hospitalized covid-19 patients during the first 12 months of the pandemic in the central city of iran.
- Seyedeh Mahideh Namayandeh
- HamidReza Dehghan
- Fatemeh Majidpour
Scientific Reports (2023)
Identification of SARS-CoV-2 Mpro inhibitors containing P1’ 4-fluorobenzothiazole moiety highly active against SARS-CoV-2
- Nobuyo Higashi-Kuwata
- Kohei Tsuji
- Hiroaki Mitsuya
Nature Communications (2023)
Implications of food and nutrition security on household food expenditure: the case of Malaysia
- Kerry Kh’ng
- Ching-Cheng Chang
- Shih-Hsun Hsu
Agriculture & Food Security (2022)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
Loading metrics
Open Access
Peer-reviewed
Research Article
The varying impacts of COVID-19 and its related measures in the UK: A year in review
Roles Conceptualization, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft
* E-mail: [email protected]
Affiliation Department of Sociology, University of Oxford, Oxford, United Kingdom
Roles Funding acquisition, Writing – review & editing
- Muzhi Zhou,
- Man-Yee Kan

- Published: September 29, 2021
- https://doi.org/10.1371/journal.pone.0257286
- Peer Review
- Reader Comments
We examine how the earnings, time use, and subjective wellbeing of different social groups changed at different stages/waves of the pandemic in the United Kingdom (UK). We analyze longitudinal data from the latest UK Household Longitudinal Survey (UKHLS) COVID study and the earlier waves of the UKHLS to investigate within-individual changes in labor income, paid work time, housework time, childcare time, and distress level during the three lockdown periods and the easing period between them (from April 2020 to late March 2021). We find that as the pandemic developed, COVID-19 and its related lockdown measures in the UK had unequal and varying impacts on people’s income, time use, and subjective well-being based on their gender, ethnicity, and educational level. In conclusion, the extent of the impacts of COVID-19 and COVID-induced measures as well as the speed at which these impacts developed, varied across social groups with different types of vulnerabilities.
Citation: Zhou M, Kan M-Y (2021) The varying impacts of COVID-19 and its related measures in the UK: A year in review. PLoS ONE 16(9): e0257286. https://doi.org/10.1371/journal.pone.0257286
Editor: Florian Fischer, Charite Universitatsmedizin Berlin, GERMANY
Received: October 13, 2020; Accepted: August 27, 2021; Published: September 29, 2021
Copyright: © 2021 Zhou, Kan. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: All data files are available from the UK Data Service database (study number(s) 6641, 8644). Dat file URL: https://beta.ukdataservice.ac.uk/datacatalogue/studies/study?id=8644 https://beta.ukdataservice.ac.uk/datacatalogue/studies/study?id=6641 .
Funding: This work is supported by the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (awardee: Man-Yee Kan, grant number 771736). Funding website: https://ec.europa.eu/programmes/horizon2020/en . The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: The authors have declared that no competing interests exist.
Introduction
More than one year has passed since the United Kingdom (UK) officially announced its first national lockdown on 23 March 2020 due to the rapid spread of COVID-19. The outbreak of COVID-19 and the massive lockdown measures have greatly changed people’s lives. When people were instructed to stay at home and maintain physical distancing, the lives of millions of people were affected. For months, many people were unable to go to work or school, nor could they meet friends and relatives. What was unexpected was that people in the UK experienced a total of three national lockdowns over the past year. Now, people’s lives are far from what they were before the first lockdown, and the pandemic is still not over.
Recent evidence has shown that the COVID-19 pandemic and related social and economic measures, such as physical distancing and business closure, have differential impacts on various social groups. In the UK, for example, women and parents are found to have experienced a larger reduction in subjective wellbeing [ 1 , 2 ]. Black, Asian, and minority ethnic (BAME) immigrants were more likely to experience economic hardship immediately after the first national lockdown [ 3 ]. In addition, among those who were known to have COVID-19, people of BAME background in the UK had a death rate that was higher than that of white people [ 4 ]. As Damian Barr said in his poem, “we are in the same storm, but we are not all in the same boat [ 5 ]”.
These earlier findings identified the existence of immediate unequal impacts for different social groups, but our understanding of the longer-term impacts of COVID-19 and related measures remains limited. We know little about how the impacts might have changed since the first lockdown. The COVID-19 pandemic has already lasted for more than one year, and the UK has experienced three national lockdowns. Early research was confined by data that covered only two time points—such as before and shortly after the announcement of the first lockdown. Little is known about to how unequal social impacts reveal themselves at different stages of the COVID-19 pandemic, especially with repeated lockdowns. This omission hinders our understanding of how COVID-19 and COVID-induced social policies, such as physical distancing measures, working from home, and the closure of certain businesses, which have been changing on a weekly or even daily basis, progressively affect people’s lives. Documenting the development of the impacts of COVID-19 and COVID-induced measures is important for us to understand the consequences of this rapidly developing pandemic and help policymakers plan for future waves and future pandemics.
We need more comprehensive and up-to-date research on how inequalities have changed as the COVID-19 pandemic develops with repeated waves and the various measures to contain it were implemented over the past year. We conducted analyses on a nationally representative population data from the latest UK Household Longitudinal Survey (UKHLS), which was conducted before the first lockdown in March 2020, during the first lockdown from April to June 2020, during the ease of the first lockdown (June to September 2020), and during the later two lockdowns (November 2020, and from January 2021 to March 2021). In this paper, we contribute to COVID-19 research by providing a dynamic picture of how people’s labor earnings, time use, and wellbeing changed across different stages of the pandemic. We further investigated whether and the extent to which the inequalities in these outcomes based on gender, ethnicity, and educational level have changed over the past year.
In what follows, we first review the latest works on the impact of COVID-19 and COVID-induced measures on people’s lives, focusing on three dimensions of social inequality: gender, race/ethnicity, and education. We then outline the development of the COVID-19 pandemic and the lockdown measures in the UK from March 2020 to April 2021. Next, we introduce the data and its longitudinal design, which enables us to compare the information of the same individuals before the start of this pandemic and at different time points over the past year. Finally, we will report the results of fixed-effect regression analyses and discuss our conclusions.
The impacts of COVID-19 and its related measures
The COVID-19 pandemic has developed for over one year. In many countries, repeated waves of COVID-19 have been observed. The primary aim of COVID-19 induced measures is to contain the virus by reducing physical contacts between people. Many of these measures immediately affect people’s behaviors, but others could have longer-term impacts. For example, the closure of businesses and work-from-home guidance tremendously altered people’s working patterns. Reductions in paid work time and earnings have been immediately recorded in countries that have introduced lockdown measures such as Australia [ 6 ], the UK [ 3 , 7 ], and the United States (US) [ 8 ]. When more people stayed at home and the option of outsourcing domestic work was reduced due to business closure or the fear of contracting COVID-19, it is not surprising to see that people spent substantially more time on unpaid domestic work than they had in the past [ 6 , 7 , 9 , 10 ].
People’s feelings also changed. The contraction of COVID-19 is associated with a series of symptoms such as a high temperature, continuous cough and a loss or change to the sense of smell or taste. Serious cases will result in hospital admission and death. In the UK, the case-fatality rate is estimated to be 2.1% [ 11 ]. Daily news reporting the surging number of new cases and deaths brings in a high level of worry about health and security [ 2 ]. In addition, loss of employment, financial strain, and social isolation are well-known factors that negatively affect mental health [ 12 – 14 ]. Not surprisingly, soon after the start of the pandemic, worsened subjective wellbeing was observed in Australia [ 6 , 15 ], the UK [ 2 , 16 , 17 ], and the US [ 18 ]. Once daily increase of COVID-19 cases declined and the lockdown restrictions began to be lifted, people’s subjective wellbeing started to recover. As Pierce et al. [ 2 ] noted by using the first five waves of the same UKHLS COVID study data as in this paper, “[b]etween April and October 2020, the mental health of most UK adults remained resilient or returned to pre-pandemic levels.” However, “[a]round one in nine individuals had deteriorating or consistently poor mental health.”
This COVID-19 pandemic and its related measures have raised increasing concerns of exacerbated social inequalities. Since long before the pandemic, gender inequalities have existed in the labor market. In the UK, the labor force participation rate for men is higher than that for women, and men are also much more likely to work full time [ 9 , 19 ]. Women are more likely to be at-home workers. Reasons for this inequality include inflexible workplace expectations, gender norms expecting men to be the primary earners and women the primary caregivers, and discrimination in the labor market. When people are required to work from home, the spatial boundary between market work and family life is blurred. Many studies have investigated whether the changes in time use due to lockdown measures are the same for women and men. Between March and May 2020 (UK 1st lockdown), British men were found to be more likely to be furloughed or dismissed from work than women [ 20 ]. However, studies focusing on the labor market performance of parents reveal a different pattern. In the UK, during the first lockdown period from April to May 2020, among parents with children aged between 4 and 15, mothers were found to be more likely to be laid off, furloughed, or quit their jobs [ 21 ]. Similarly, in Australia [ 6 ], Canada [ 22 ], and the US [ 23 ], mothers with young children experienced a larger change in their paid work time or were more likely to leave their jobs. On the other hand, several studies have reported improvements in the domestic division of labor: the increase in domestic work was larger for men than for women during the lockdown period in Australia [ 6 ], Canada [ 24 ], France [ 25 ], and the US [ 26 ]. However, contrary results were reported in Germany [ 27 ] and Spain [ 28 ]. The decline in subjective wellbeing also differs between women and men. In the UK and Australia, women were found to experience a larger reduction in subjective wellbeing than men [ 1 , 2 , 6 , 9 , 29 ].
In the UK, BAME immigrants were more likely to experience economic hardship just after the first lockdown [ 3 ]. Compared with their white counterparts, BAME immigrants were also found to suffer a larger decline in subjective wellbeing at the beginning of the March 2020 lockdown in the UK [ 3 , 30 ]. In the US state of Indiana, Black Americans were more than three times more likely to lose their jobs than whites [ 31 ]. In contrast, another study highlights that white Britons in middle-income jobs were more likely to experience job loss, primarily driven by the fact that many BAME people are employed in key sectors such as the health and social care services, which were exempt from the lockdown measures and instead had a surge in work demands, during the first UK lockdown [ 20 ]. Notably, in the UK, people of BAME backgrounds had a death rate that was higher than that of white people after they were confirmed to have COVID-19 [ 4 ].
People with less education and lower income suffered substantially during the pandemic. They were particularly hit hard with a higher chance of losing their jobs and earnings in countries such as Canada [ 32 ], the UK [ 20 ], and the US [ 31 ]. Many of the less educated are trapped in lower-skilled occupations with tight financial constraints. Consequently, the less educated group reported a heightened level of distress during the first lockdown in the UK [ 33 ]. However, one US study reports that the decline in subjective wellbeing up to April 2020 was larger among the more educated, possibly because the more educated might have felt a greater loss of control and wealth due to COVID-19-related uncertainties [ 18 ]. Another study conducted in the US between April 2020 and June 2021 pointed out that part of the reason for the deterioration of mental health results should be attributed to the concurrent presidential election and unrest in domestic politics [ 34 ].
Again, the current literature has focused extensively on the impacts of the relatively early stage of this pandemic. In particular, studies that have employed the same British data source as the present study have examined the changes in earnings, time use, and subjective wellbeing during the implementation of the first national lockdown in late March 2020 [ 3 , 7 , 9 , 10 , 20 ]. Pierce et al.’s work [ 2 ] on subjective wellbeing is an exception. Their work examined the recovery of subjective wellbeing when the first lockdown measures were eased from June to October 2020. However, their study did not cover the later lockdowns in November 2020 and January 2021. In this article, we will provide a first-year review of COVID-19 development in the UK and document how people have responded to the first lockdown, the ease of the first lockdown, and the later two lockdowns. This evaluation will reveal whether people responded similarly to repeated lockdowns and whether these changes in earnings, time use, and feelings are temporary or long-lasting.
Timeline of the lockdown measures in the UK
On 31 January 2020, the first two positive cases of COVID-19 were confirmed in the UK. On 5 March 2020, the first patient who tested positive for COVID-19 died. On 23 March 2020, the Prime Minister placed the UK on lockdown to slow down the outbreak of this pandemic. These measures included physical distancing, school closures, working from home, and closure of non-essential businesses, including pubs and cafes. Key sectors, including health and social care, education and childcare, and key public services, were allowed to operate.
To maintain employment and to protect individuals and businesses from economic hardship, a coronavirus job retention scheme was implemented for the period between late March and the end of October 2021 to cover 80 percent of the regular salary of furloughed employees, up to a maximum of £2,500 per month [ 35 ]. In April, the UK had more than 10,000 deaths related to COVID-19. In May, phased reopening of shops and schools was announced, and those who were unable to work from home were expected to return to the workplace.
Beginning on 1 June 2020, schools were open for all Reception, Year 1 and Year 6 pupils, but the summer holiday soon arrived. Nonessential businesses reopened gradually beginning on 15 June. Beginning on 4 July, pubs, cinemas, restaurants reopened. Physical distancing rules were relaxed from a “two-meter” to a “one-meter plus” rule. In August, restrictions were eased further, although the pandemic was far from over.
The UK variant of the coronavirus (scientific name B.1.1.7, WHO name Alpha) was first identified in September 2020 and was considered to be more transmissible and potentially deadlier. In late September, people were required to work from home with a 10 pm curfew for the hospitality sector. In October, England entered a 3-tier system where different regions were classified into different tiers depending on the level of the spread of the virus. Soon after, the second national lockdown came into force on 5 November and lasted until 2 December. People were told to stay at home. Other measures included the closure of the hospitality sector and nonessential shops, but schools were open, and people could leave their home for outdoor exercise. After 2 December, the UK then entered a stricter 3-tier restriction system.
However, this 3-tier system did not last long. After Scotland announced a lockdown, on 4 January 2021, a third national lockdown was announced. Schools were closed again, and people were urged to stay at home. This time, the measures were stricter than those in the second lockdown. They included “Stay at home at all times, wherever possible,” “Not allowed to meet others from outside your household (or support bubble),” “All retail and hospitality venues must close,” and “Personal care services have to close.” Schools were closed to most pupils, except for the children of critical workers and the most vulnerable children. Nurseries were kept open.
Since 8 March, schools in the UK have been completely reopened. Nonessential retail and personal care services have been reopened since 12 April. People have been allowed to meet outdoors, as a number of restrictive measures have been lifted since 17 May. A complete easing will occur on 19 July 2021. The Prime Minister has pledged that all adults in the UK will be offered their first dose of a COVID-19 vaccine by the end of July.
By 16 April 2021, the recorded number of deaths related to COVID-19 had reached over 127,000 in the UK. Fig 1 displays the spread of COVID-19 and related deaths in the UK during the research period. A more detailed timeline of the UK lockdowns can be found at [ https://www.instituteforgovernment.org.uk/sites/default/files/timeline-lockdown-web.pdf ]. Fig 1 shows the development of the COVID-19 pandemic in the UK based on data provided by the UK government.
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
Note: Data source: https://coronavirus.data.gov.uk/details . Crude death rate is new deaths within 28 days of a positive test per 100,000 population.
https://doi.org/10.1371/journal.pone.0257286.g001
Data and methods
Data and sample.
We use data from the first eight waves of the UKHLS COVID study data and the preceding two waves (2017/18 and 2018/19) of the UKHLS main survey [ 36 ]. The UKHLS is a household panel survey and started its first wave in 2009 with a nationally representative sample of 51,000 adults (aged 16 and above) from approximately 40,000 households. Individuals were followed up annually and were interviewed face-to-face. This research is based completely on the UKHLS data that are publicly available through the UK Data Service (Study numbers: 6614 and 8644) and are completely anonymous.
Regarding the COVID study, households who participated in previous UKHLS surveys were contacted to fill in a monthly online questionnaire beginning in April 2020. The complementary telephone survey started in May 2020. Participation in the survey was voluntary. Approximately 16,000 respondents (aged 16 and above) completed this first wave of the COVID survey with a response rate of 42%. Currently, data from the first eight waves of surveys conducted in the last week in April, May, June, July, September, November in 2020 and the last week in January and March in 2021 are available.
Our analytic sample contains individuals who have participated in the UKHLS main survey and at least one of the eight waves of the COVID study. The respondents all had access to the internet or telephone to participate in the surveys. This requirement might have caused a sample selection bias. In a supplementary analysis, the sample from the COVID study is found to be socioeconomically advantaged in terms of employment, occupation, education, and homeownership compared to the full UKHLS sample. If we assume that one’s socioeconomic status has a protective effect on the negative consequences of the COVID-19 and related lockdown measures, the reported results may underestimate the potential negative impacts of the COVID-19 and the related lockdown. Nonetheless, one paper discusses this issue of nonrandom sample selection and demonstrates that the bias due to sample selection is very limited once weight is considered [ 37 ]. In the following analysis, we apply the individual weights, which were adjusted for “unequal selection probabilities and differential nonresponse” and are supplied in the data [ 38 ]. Based on the User Guide for the data, these weights “scale respondents to the eligible population in the UKHLS wave 9 sample, adjusted for death, incapacity and emigration occurring between wave 9 and the start of the COVID-19 web survey.” [ 38 ] This approach has been used in previous work analyzing the same data [ 2 , 3 , 20 ].
Our sample includes respondents of prime working age (between 20 and 65) in 2020. Two percent of the UKHLS COVID sample has missing values in the predictors to be used in regressions. The numbers of observations with no missing predictors are 10484, 9008, 8478, 8210, 7642, 7083, 7019, and 7525 in the first eight waves of the COVID study. The final sample for each regression is dependent on the outcome variables with nonmissing values (some outcome variables are not asked in certain waves) and the selection of subgroups (for example, people who had a job before the pandemic). Please refer to S1 Table for more details of the sample selection process. The focus on within-individual changes in the outcome variables indicates that the respondents should be followed up for more than one wave. Previous analyses using the same data and selecting the individuals interviewed for more than one wave do not find that this selection would bias the results [ 39 ].
Monthly labor income, weekly paid work hours, subjective wellbeing, weekly housework hours, and weekly childcare hours are the five dependent variables or outcomes of interest.
Monthly labor income.
Respondents’ labor income in January or February 2020 (before the lockdown) was collected retrospectively in the COVID survey. Respondents also provided their current labor income in each month thereafter. We calculate the natural log of the labor income. Those who had a job in January or February 2020 were selected to predict this outcome.
Weekly paid work hours.
Respondents retrospectively reported their current paid work hours per week and their usual working hours in January or February 2020. During the period of the COVID-19 pandemic, the question asked was “How many hours did you work, as an employee or self-employed, last week?” During the prepandemic period, the question was “During January and February 2020, how many hours did you usually work per week?” Those who had a job in January or February 2020 were selected to predict this outcome.
Subjective wellbeing.
Subjective wellbeing is the mental wellbeing reported by the respondents in a General Health Questionnaire (GHQ). The value is the sum of 12 items (GHQ-12) scored on a Likert scale from 0 to 3: “ability to concentrate,” “losing sleep,” “playing a useful role in life,” “capability of making decisions,” “feeling under stress,” “overcoming difficulties,” “ability to enjoy activities,” “ability to face problems,” “feeling unhappy or depressed,” “losing confidence,” “believing in self-worth,” and “feeling generally happy.” The overall scale ranges from 0 (least distressed) to 36 (most distressed). This measurement is a validated and widely used measure of nonspecific mental distress in surveys [ 40 ]. The same information was collected in earlier waves of the main survey of the UKHLS and in each wave of the COVID study. The full sample was used to predict this outcome.
Weekly housework hours.
Respondents’ weekly housework hours were collected by the question “Thinking about last week, how much time did you spend on housework, such as time spent cooking, cleaning and doing the laundry?” Information about housework hours before the COVID survey was derived from the earlier UKHLS waves (the latest one was collected in the years between 2018 and 2019). The full sample was used to predict this outcome.
Weekly childcare hours.
Respondents’ childcare hours were collected by the question “About how many hours did you spend on childcare or home-schooling last week?” This information is only available in the COVID survey. Only those who had a child younger than 16 years old in the household (referred to as parents in later analyses) were asked this question, and these respondents are used for analyses.
Independent variables.
We include the wave dummies, which represent the time point when information was collected to examine the dynamics in those outcome variables.
The key socioeconomic independent variables are constant for the same individual across the waves. These variables are gender (52.7% females), whether an individual is Black, Asian or another minority ethnic (10.1%) or not (reference group: whites), and educational level (university degree holders 32.2%). The underrepresentation of ethnic minority groups is common in a panel survey sample (the 2011 census reported that 85.6% of the working-age people were from white ethnic groups) because of the selection of people with repeated observations to satisfy the requirement of the fixed-effect models. People with disadvantaged backgrounds are known to be more likely to drop out in repeated surveys [ 41 ]. The later regression analysis has considered this sample selection issue using weights, as discussed above. Moreover, attrition in panel surveys is not found to have a significant impact on the estimations in predicting income [ 42 ], time use [ 43 ], or attitudes [ 44 ].
Whether the respondent had a positive COVID-19 test outcome was asked in each wave. We included this variable in the model to control for the impact of contracting COVID-19 so that the period indicators could better represent the spread of COVID-19 and COVID-19-related policy change at the macro-level. This variable has four categories: “having no test” (reference, 89.7%), “tested positive” (0.8%), “tested negative” (9.0%), and “result pending” (0.5%).
All models controlled for respondents’ partnership status (whether they live with a partner) and parenthood status (the presence of a child younger than age 16 in the household) to account for potential changes in the family status that are correlated with the outcomes [ 45 , 46 ].
Analytical strategies
We applied linear fixed-effect regressions to predict the five outcomes. By interacting the month indicator with gender, BAME group, and education levels, we examined how the change in income, time use, and wellbeing differed across individuals in the three different sociodemographic groups in different periods of the pandemic. The reference time point is January and February 2020 for earnings and weekly paid work hours outcomes. The reference time point is the year 2018/2019 for the subjective wellbeing (distress level) and weekly housework hours outcomes. For weekly childcare hours, the reference time point is April 2020, which was during the first national lockdown. The outcome variables compare the information reported by the same individuals at each time point and hence reveal within-person changes. This analytic approach enabled us to investigate trajectories of the outcome variables over the past year conditional on the same individual.
The fixed-effect regression method takes full account of the time-constant individual characteristics that are correlated with both the independent variable and the outcome variables. This is achieved by demeaning the dependent and independent variables using person-specific means [ 47 ].
The samples in the UKHLS main survey and the COVID survey are probability samples of postal addresses. The samples are clustered and stratified. Accordingly, clustered standard errors are used to consider this sampling design [ 48 ].
These analyses were conducted in Stata/SE 16.1. Replication codes are available at https://github.com/jomuzhi/ukcovidunderstandingsociety .
Descriptive results
We first report the weighted mean values of the key outcomes in Table 1 . Please note that the information was collected at the end of each survey month.
https://doi.org/10.1371/journal.pone.0257286.t001
First, among those who worked before this pandemic (between January and February 2020), there was a clear reduction in their average earnings when the pandemic started in the UK. Their income recovered by almost ten percent in May from the April level, which should have been mainly driven by the implementation of the job retention scheme . Some workers who could not work from home, such as those working on construction sites, also returned to the workplace in May. Since then, average monthly net earnings have remained at approximately the level of £1,550. Notably, since the first lockdown, people’s take-home earnings has never returned to their prepandemic level but never fell below 90% of the pre-pandemic level.
Before the pandemic, those who worked in January and February 2020 worked 34.7 hours per week on average. A record low of 21.9 hours per week was observed in April 2020. The persistent decline in paid work time over the past year is evident, although working hours have recovered gradually since May and reached a peak of approximately 30 hours per week in September 2020. The later two national lockdowns (November 2020 and January 2021) did not reduce the working hours as much as the first national lockdown. Weekly paid work hours were maintained at approximately 28 hours.
People felt more distressed beginning in March 2020. The worst number of 13.4 was recorded in the last two rounds of lockdown-November 2020 and January 2021, when new cases and deaths grew sharply at the beginning of these lockdowns.
People’s housework hours increased and reached the highest level of 12.3 hours per week in April and May 2020. Then, housework time declined gradually and was maintained at 10.5 hours per week. Compared with the figure recorded in September 2020 when most lockdown restrictions were eased, the figure in January 2021 did not change significantly, even though a stricter lockdown was in place. This finding concurs with the small reduction in paid work hours from September 2020 to January 2021.
The average childcare hours per week reached 16.7 hours for parents in April, but this figure gradually declined to approximately 13 hours per week before the third national lockdown. In January 2021, childcare hours only increased 0.5 hours per week over the September figure, even though schools were closed to most pupils during the third lockdown. Overall, people’s time use had become less responsive to repeated lockdowns.
Changes in earnings, paid work time, subjective wellbeing, housework and childcare time
Fig 2 reports within-individual changes in earnings, paid work hours, distress level, and housework hours across waves. The red lines indicate the time point when the national lockdowns started to enforce. Please note that the information was collected at the end of each survey month. Detailed coefficients are reported in S2 Table .
https://doi.org/10.1371/journal.pone.0257286.g002
Respondents’ earnings stayed lower than the pre-pandemic level over the entire year, with the largest decline (~9%) recorded in late April, the first month after the announcement of the first national lockdown. Earnings recovered slightly after the gradual relaxation of restrictive measures and the implementation of the job retention scheme. Following the third lockdown, when almost the same strict measures as the first lockdown were imposed, we found a similar level of decline in earnings (~8%) compared with the prepandemic period, as in the first lockdown. One year after the onset of the pandemic in the UK, our sample still experienced a 7.4% decline in earnings compared with the pre-pandemic level.
Paid work hours remained much lower than the prepandemic level over the entire year. The largest drop of nearly 13 hours was observed in the first month after the March 2020 lockdown. Then, paid work hours recovered and have never returned to the same lowest point. People worked the longest hours in September 2020, when restrictive measures were minimal. Interestingly, despite the implementation of the second and the stricter third national lockdowns, paid work hours dropped only slightly compared to the September figure and were even higher than the July 2020 figure, even though all shops were allowed to open back in July 2020. This observation suggests an increased adaptation to the work-from-home practice. After the first lockdown, more firms announced a long-term strategy to allow employees to work from home [ 49 ]. Accordingly, people have increased their paid work time even though they might still work from home.
In this pandemic, people’s subjective well-being has been damaged. The distress level (a higher score indicating more distress) stayed higher than the prepandemic level over the past year. In the three-month period after the first lockdown, a high level of distress was recorded. An improvement in subjective wellbeing was observed from July and before the enforcement of the second lockdown. The November lockdown brought a further decline in subjective wellbeing, which is consistent with the findings in one earlier study [ 2 ]. The distress level in November 2020 and January 2021 was even higher than that in the first lockdown period. It appears that people were much less optimistic and suffered tremendously as the pandemic dragged longer. People became slightly less negatively affected in their subjective wellbeing in March 2021, although the level was only similar to that in April 2020. One year after the onset of the pandemic in the UK, respondents’ subjective wellbeing returned to the level of April 2020, which was one month after the announcement of the first national lockdown.
The increase in housework hours was the highest during the first lockdown. Compared with the housework hours during the easing period in September 2020, the January 2021 lockdown was not associated with an increase in people’s housework time. This change echoes the relatively high level of paid work time in the later two lockdown periods.
Next, we examine childcare time since the first national lockdown. In Fig 3 , we can see that beginning in April 2020 (during the first lockdown period), childcare hours have been dropping. The lowest level was observed in September 2020, when schools completely reopened. Interestingly, childcare hours in January 2021 were similar to those in September 2020, despite the closure of schools to most children in January 2021.
https://doi.org/10.1371/journal.pone.0257286.g003
Differential impacts on women and men
Figs 4 and 5 report whether changes in the five indicators differ between women and men. For monthly net earnings and weekly paid work hours, we analyzed an additional sample that includes only non-key workers. We will examine whether a disproportionate number of female workers in certain key sectors, such as health and social care, drive the results.
https://doi.org/10.1371/journal.pone.0257286.g004
https://doi.org/10.1371/journal.pone.0257286.g005
First, the reduction in earnings for female workers (those who worked in Jan/Feb 2020) was smaller than that for male workers during the first lockdown in April 2020 (p = 0.011). Since then, there has been no difference between women and men in changes in earnings, reflecting the faster recovery of men’s earnings. Differential impacts on women and men were not found among non-keyworkers. Therefore, the higher proportion of women working in key sectors, which were operating much more actively than other sectors during the first lockdown period, should be the main reason for the gender difference in the earning decline during the first lockdown.
During the first lockdown, the decline in paid work hours was smaller for female workers than for male workers, disregarding their keyworker status (p<0.001). The gender difference in the reduction in paid work hours decreased as the first lockdown ended and became statistically insignificant at the 0.05 level from July to September 2020, indicating a faster recovery of paid work time for men than for women. The differential impacts of gender on paid work hours observed in the first lockdown were not observed in later lockdowns among non-keyworkers.
In Fig 5 , the growth in distress level was much higher for women than for men in the first month of the first lockdown (p<0.001). Then, women’s subjective wellbeing recovered, and men’s distress levels began to rise. These findings suggest that men’s response to this pandemic lagged behind that of women in terms of their subjective wellbeing in the first lockdown. The distress level of both women and men was reduced to the lowest level from July to September 2020, when life in general had returned to normal. Once the cases of COVID-19 surged and lockdown restrictions were reimposed in November 2020 (p = 0.056) and January 2021 (p = 0.061), women again suffered from a larger increase in distress levels than men. The distress level of women reached a similar high point across the three lockdowns. For men, their distress level was higher in the later lockdowns than in the first lockdown, when the cases of COVID-19 and its related deaths worsened.
We do not observe a gender-specific impact on housework time. The gender gap in housework time was maintained over the past year.
Differential impacts on BAME people and white people
Figs 6 and 7 report whether changes in the five indicators differ between BAME people and whites. For monthly net earnings and weekly paid work hours, we analyzed an additional sample that includes only non-key workers.
https://doi.org/10.1371/journal.pone.0257286.g006
https://doi.org/10.1371/journal.pone.0257286.g007
Compared with whites, the earnings of the BAME group were particularly negatively affected by the pandemic. The differential impacts on earnings persisted across almost all months over the past year, except during the third lockdown. The gap was large even when most lockdown restrictions were eased in September 2020 (p = 0.003). The earning gap between the BAME group and whites was even larger among non-key workers. Over the past year, the decline in market working time was similar for the BAME group and whites in both the full and the non-key worker samples. In March 2021, the reduction in paid work time decreased less for the BAME group than for the whites (p = 0.006).
Regarding the distress level ( Fig 7 ), the increase for the BAME group was larger than that for whites during the first lockdown, but the difference was not statistically significant at the 0.05 level due to the large standard error of the estimates of the BAME group. Beginning in September 2020, the changes in the distress levels were similar for the BAME group and whites. The increase in housework hours seems to be larger for the BAME group, but the large standard errors prevent us from drawing a reliable conclusion.
Differential impacts on degree and non-degree holders
Figs 8 and 9 report whether changes in the five indicators differ between degree and non-degree holders. For monthly net earnings and weekly paid work hours, we analyzed an additional sample that includes only non-key workers.
https://doi.org/10.1371/journal.pone.0257286.g008
https://doi.org/10.1371/journal.pone.0257286.g009
As expected, the decline in earnings and paid work hours was particularly acute among non-degree holders. These differential impacts were even larger among non-key workers. When the spread of the virus decreased and most of the restrictive measures eased from July to September 2020, the difference in the impacts on non-degree and degree holders became smaller but was sustained. For paid work hours, the difference was insignificant between July and September 2020 for both the full and the non-keyworker samples. Once restrictive measures were reimposed, the difference became substantial again (p<0.001).
As Fig 9 shows, there was no significant difference in the change in subjective wellbeing between degree and non-degree holders before January 2021. However, degree holders experienced a larger increase in distress level during the third national lockdown that started in January 2021 (p = 0.028), but the differential effect disappeared in March 2021.
We do not observe a statistically significant difference in the changes in housework time between the two groups.
Changes in weekly childcare hours since April 2020
Fig 10 reports whether changes in the weekly childcare hours differ across these groups.
https://doi.org/10.1371/journal.pone.0257286.g010
Our findings show that women and men, BAME people and whites, and degree and non-degree holders did not differ significantly in changes to their childcare time since April 2020. However, there is a tendency that the reduction in childcare time in September, which should be associated with pupils returning to schools after summer vacation, was larger for mothers and the more educated group, suggesting that women and the more educated might have spent more time taking care of children at home.
For more details of the results, please refer to S2 – S5 Tables. The within-individual R-squares are small when predicting earnings, subjective wellbeing, housework time, and childcare time. Small within-individual R-squares are not uncommon in fixed-effect regressions, especially when predicting housework time and subjective wellbeing [ 50 , 51 ]. These results suggest that a limited number of individuals have changed their partnership and parenthood status and COVID-test results, but their outcome variables—earnings, time use, and subjective wellbeing-have changed considerably over the past year. The inclusion of more time-varying variables might be able to improve the explanatory power. Those variables could be whether furloughed, whether participated in the job retention scheme, or whether went back to work/school. However, the purpose of this paper is to provide an overall net impact of COVID-19 and its related measures on an individual instead of focusing on a specific policy or the spread of COVID-19. Given the focus on the trajectories of earnings, time use, and subjective wellbeing at different stages of the pandemic, we do not include those time-varying variables suggested above.
Discussion and conclusion
In this article, we have utilized the latest UK COVID panel data to provide a comprehensive analysis of the dynamics of earnings, time use, and subjective wellbeing at different stages of the pandemic over the past year. Our research, with a much extended time scope, surpasses past UK studies that only followed a short period after the first lockdown imposed in March 2020 [for example, 3, 7, 9, 20]. Our analysis has incorporated multiple domains of outcomes across several social groups. We aim to examine how the spread of COVID-19 and COVID-induced policies have had unequal and dynamic impacts on different social groups in the UK. Our findings offer important insights into whether inequalities in changes in income, time use and wellbeing are likely to be long lasting or temporary.
Overall, the initial outbreak of COVID-19 and the first national lockdown brought the largest change in earnings and time use. The later two lockdowns together with the repeated new highs of the COVID-19 cases and deaths impacted people’s subjective wellbeing the most. Although strict measures that aimed to reduce people’s physical contact were imposed in the later two lockdowns, people’s time use did not respond as strongly as they did during the first lockdown. Among the five indicators, none had returned to their prepandemic level until late March 2021. It remains uncertain when and whether earnings, working patterns, family life, and subjective wellbeing will return to the prepandemic level.
Female workers experienced less reduction in their earnings than male workers, which is largely due to the relatively high proportion of women working in key sectors, especially in the health and social care industry. Women have made an important contribution to the fight against COVID-19 by working in key sectors. However, even among non-key workers, the decline in paid work hours was smaller for women but only during the first lockdown period. These findings concur with earlier research that reported that men in the UK were more likely than women to be laid off or furloughed during the first lockdown [ 20 ]. Once lockdown measures were gradually lifted beginning in June 2020, men’s paid work time recovered faster than that of women. This finding is similar to previous work on the gendered impact of natural disasters on market labor [ 52 ]. In summary, our analysis has shown that in the UK, men’s paid work time was more responsive to the restrictive measures of the first lockdown, but women’s and men’s paid work time responded similarly in the later two lockdowns.
The subjective wellbeing of women was more sensitive to the outbreak of COVID-19 and related lockdown measures than that of men. For example, the increase in women’s distress level was substantial in April, but it then gradually improved until the next lockdown. Men’s responses lagged behind of those of women. Past COVID-19 research has highlighted the gender difference in social networks, where women tend to have more friends [ 29 ]. The larger exposure to news related to COVID-19 for those with more close friends might be the factor that explains the diverging trajectories of women’s and men’s subjective wellbeing [ 53 , 54 ]. Theses differential impacts became smaller in later two lockdowns, as the pandemic had developed for a certain period. At the beginning of the pandemic, women and men seemed to have perceived the danger of this infectious disease differently.
The gender gap in housework time was maintained over the past year. Overall, the gender-specific changes in earnings, paid work time, and subjective wellbeing were mainly observed when strict restrictions were in place, and the gender gap returned to its prepandemic level once those measures were lifted.
People of a BAME background experienced a larger loss in earnings than whites. This finding is consistent with an earlier finding on BAME immigrants in the UK [ 3 ]. We have further shown that the enlarged earning gaps between BAME and white people persisted almost over the entire year.
Persistently enlarged earning gaps were observed between non-degree and degree holders. The gap was even larger among non-key workers. Non-degree holders suffered from a larger reduction in earnings across all months over the past year. This gap was particularly large during the national lockdown periods. A similar observation was found for weekly paid work hours. The spread of COVID-19 and lockdown restrictions are associated with an enlarged gap in paid work time between non-degree and degree holders. This effect on paid work time is likely to be temporary because differential impacts were not observed from July to September 2020, when lockdown measures were mostly lifted.
One limitation of this study is that some changes could be brought by seasonal fluctuations beyond COVID-19 and its related restrictions. For example, people’s paid work time in winter may differ from that in summer. General psychological health was usually worse in winter than in summer [ 55 ]. The ideal solution is to compare information collected in the same month before the pandemic and in 2020. However, this approach is not possible with the current data. If the current survey retains the current monthly or bimonthly data collection frequency, future work can compare the same month in 2020 and the years after to examine pandemic and post-pandemic differences. We have also included the measure of the spread of COVID-19 (daily new cases or daily new death rates, as shown in Fig 1 ) to examine whether the outcomes are affected by the macrolevel development of the COVID-19 pandemic in the UK. We do not find strong evidence showing that those measures are associated with the outcomes. Our results reveal the trajectories of earnings, time use, and subjective wellbeing at different time points over the past year but cannot identify the exact impact of a specific lockdown restrictive policy. There could be other non-COVID-19-related policy updates that occurred in parallel over the past year that may have had an impact on the same outcomes. Nonetheless, the trends of the observed changes in income, time use, and subjective wellbeing corresponded closely to the different waves of the pandemic and the lockdown timeline. Therefore, the major sources of those changes should be related to the spread of COVID-19 and its related lockdown measures.
In conclusion, our findings suggest that the long-lasting pandemic and the related restrictions to contain the virus over the past year have produced persistent negative consequences for earnings, work patterns, and subjective wellbeing. The spread of COVID-19 and the national lockdowns at different stages had distinct patterns and measures, and their impacts on labor earnings, time use and subjective well-being varied. Time use patterns became less sensitive to the later lockdowns, but the distress levels reached a new high with repeated lockdowns in multiple waves of the pandemic. The differential impacts of the lockdown measures based on gender became insignificant once lockdown measures were lifted. However, some social groups, including BAME and white people and non-degree holders and degree holders, experienced persistently enlarged gaps in earnings. The negative impacts of the spread of COVID-19 and its related measures vary not only in their extent but also in their speed among different social groups. Further research should be conducted to understand factors that have driven these social inequalities and to monitor how inequalities based on gender, educational level, and ethnic minority status might be persistent or even exacerbated in the long term.
Supporting information
S1 table. samples and sample selection..
https://doi.org/10.1371/journal.pone.0257286.s001
S2 Table. Baseline model: Changes in the five indicators across waves.
https://doi.org/10.1371/journal.pone.0257286.s002
S3 Table. Gender and period interaction model results.
https://doi.org/10.1371/journal.pone.0257286.s003
S4 Table. Ethnicity and period interaction models.
https://doi.org/10.1371/journal.pone.0257286.s004
S5 Table. Education and period interaction model results.
https://doi.org/10.1371/journal.pone.0257286.s005
- View Article
- Google Scholar
- PubMed/NCBI
- 5. Barr D. Same storm, different boats. 2020. Available: https://www.damianbarr.com/latest/https/we-are-not-all-in-the-same-boat
- 11. Coronavirus Resource Center, Johns Hopkins. Mortality analysis. 2021. Available: https://coronavirus.jhu.edu/data/mortality
- 19. Office for National Statistics. Full report—women in the labour market: 2013. Office for National Statistics. 2013. Available: http://www.ons.gov.uk/employmentandlabourmarket/peopleinwork/employmentandemployeetypes/articles/womeninthelabourmarket/2013-09-25
- 29. Etheridge B, Spantig L. The gender gap in mental well-being during the covid-19 outbreak: Evidence from the UK. Colchester: University of Essex, Institute for Social; Economic Research (ISER); 2020. Report No.: 2020–08. Available: http://hdl.handle.net/10419/227789
- 30. Giovanis E. Income losses and subjective well-being: Gender and ethnic inequalities during the Covid-19 lockdown period in the UK. Research Square; 2021. https://doi.org/10.21203/rs.3.rs-132600/v2
- 34. Blanchflower DG, Bryson A. Biden, COVID and mental health in America. NBER Working Paper. 2021. https://doi.org/10.3386/w29040
- 35. UK Government3. Check if your employer can use the coronavirus job retention scheme. GOV.UK. 2020. Available: https://www.gov.uk/guidance/check-if-you-could-be-covered-by-the-coronavirus-job-retention-scheme
- 36. Institute for Social and Economic Research, University of Essex. 2020. https://doi.org/10.5255/UKDA-SN-8644-3
- 38. Institute for Social and Economic Research. Understanding Society COVID-19 user guide. Version 8.0. Colchester: University of Essex. 2021.
- 39. Uhrig SCN. The nature and causes of attrition in the British Household Panel Study. Colchester: University of Essex, Institute for Social; Economic Research (ISER); 2008. Report No.: 2008–05. Available: http://hdl.handle.net/10419/92025
- 47. Allison PD. Fixed effects regression models. SAGE Publications; 2009. Available: https://books.google.co.uk/books?id=3UxaBQAAQBAJ
- 48. Abadie A, Athey S, Imbens GW, Wooldridge J. When should you adjust standard errors for clustering? National Bureau of Economic Research, Inc; 2017 Nov. Report No.: 24003. Available: https://ideas.repec.org/p/nbr/nberwo/24003.html
- 50. Borra C, Browning M, Sevilla Sanz A. Marriage and housework. IZA Discussion Paper. 2017. Available: https://ssrn.com/abstract=2960549
- 52. Bradshaw S. Socio-economic impacts of natural disasters: A gender analysis. United Nations Economic Commission for Latin America and the Caribbean. 2004. Available: https://www.cepal.org/en/publications/5596-socio-economic-impacts-natural-disasters-gender-analysis
- Article Information
A, Error bars represent 95% CIs of the excess death estimates. For reference, excess deaths in the US general population over the same period were included. B, The shaded area represents the 95% CI of the excess death estimate.
eReferences
Data Sharing Statement
See More About
Select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Others Also Liked
- Download PDF
- X Facebook More LinkedIn
Kiang MV , Carlasare LE , Thadaney Israni S , Norcini JJ , Zaman JAB , Bibbins-Domingo K. Excess Mortality Among US Physicians During the COVID-19 Pandemic. JAMA Intern Med. 2023;183(4):374–376. doi:10.1001/jamainternmed.2022.6308
Manage citations:
© 2024
- Permissions
Excess Mortality Among US Physicians During the COVID-19 Pandemic
- 1 Epidemiology and Population Health, Stanford University School of Medicine, Stanford, California
- 2 American Medical Association, Chicago, Illinois
- 3 PRESENCE Center, Stanford University School of Medicine, Stanford, California
- 4 Department of Cardiovascular Medicine, University of Southern California, Los Angeles
- 5 Editor in Chief, JAMA and the JAMA Network, Chicago, Illinois
In the US, the COVID-19 pandemic has already resulted in over 1 million excess deaths, 1 defined as the difference between the number of observed and expected deaths over a specified period. 2 Despite their essential role in the pandemic response, little is known about excess deaths among physicians. Using data from the American Medical Association (AMA), we calculated excess deaths from March 2020 through December 2021 among US physicians.
Using the AMA Masterfile and corresponding Deceased Physician File, we fit quasi-Poisson models, accounting for within-year seasonality and long-term trends, 3 to estimate monthly mortality from January 2016 through February 2020 among physicians aged 45 to 84 years. We used this counterfactual model to estimate expected deaths from March 2020 through December 2021, and we calculated differences between observed and expected deaths to identify excess deaths. Excess mortality estimates were annualized to excess deaths per 100 000 person-years. We analyzed the total sample by age group and type of practice. We fit alternative model specifications in sensitivity analyses. For comparison, we calculated excess deaths in the US general population (eMethods in Supplement 1 ). We excluded younger physicians because this group experienced fewer than 5 deaths per month during the period of interest. All analyses were performed using R 4.2.1 (R Foundation for Statistical Computing). The Stanford University Institutional Review Board approved the study. The Stanford Administrative Panels on Human Subjects Research waived the informed consent requirement for various reasons. We followed the STROBE reporting guideline.
From March 2020 through December 2021, there were 4511 deaths (representing 622 [95% CI, 476-769] more deaths than expected) among a monthly mean (SD) of 785 631 (8293.5) physicians. These physicians consisted of 34.7% females and 65.3% males aged 45 to 84 years ( Table ). There were 43 (95% CI, 33-53) excess deaths per 100 000 person-years.
There was a strong age gradient among active physicians providing direct patient care, with excess deaths per 100 000 person-years of 10 (95% CI, 3-17) in the youngest group and 182 (95% CI, 98-267) in the oldest group ( Figure , A). Within all age groups, physicians had substantially lower excess mortality than the general population ( Figure , A). Nonactive physicians had the highest excess deaths per 100 000 person-years (140; 95% CI, 100-181) compared with active physicians providing direct patient care (27; 95% CI, 18-35) and active physicians not providing direct patient care (22; 95% CI, –8 to 51) but a substantially lower excess mortality rate than the general population (294; 95% CI, 292-296).
Among all active physicians, excess deaths peaked to over 70 in December 2020 and then had a rapid monotonic decrease in 2021. There was no statistically significant excess mortality after April 2021 ( Figure, B). These results were robust to alternative model specifications.
From March 2020 through December 2021, US physicians experienced 622 more deaths than expected. There were no excess deaths among physicians after April 2021, coinciding with the widespread availability of COVID-19 vaccines. Across age groups, physicians had substantially lower excess mortality than the general population; however, active physicians had lower excess mortality than nonactive physicians despite their higher risk of contracting SARS-CoV-2 infection. 4 The findings suggest that personal protective equipment use, vaccine requirements, infection prevention protocols, adequate staffing, and other workplace-based protective measures were effective in preventing excess mortality. Additionally, increased excess deaths among older active physicians providing direct patient care suggest that workplace policies should prioritize mitigating risk in this group.
Study limitations include unidentified physician deaths and pandemic-related workforce changes, such as early retirement among older physicians, which would result in underestimation of mortality. During the first year of the pandemic, US physicians experienced excess mortality in addition to increased workplace stress and burnout. 5 During COVID-19 surges, these conditions may strain hospitals, resulting in excess deaths in the general population. 6 Preventing excess deaths among physicians is an important component of mitigating excess deaths in the general population.
Accepted for Publication: November 20, 2022.
Published Online: February 6, 2023. doi:10.1001/jamainternmed.2022.6308
Open Access: This is an open access article distributed under the terms of the CC-BY License . © 2023 Kiang MV et al. JAMA Internal Medicine .
Corresponding Author: Mathew V. Kiang, ScD, Department of Epidemiology and Population Health, Stanford University School of Medicine, 1701 Page Mill Rd, MC: 5560, Palo Alto, CA 94304 ( [email protected] ).
Author Contributions: Dr Kiang had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Kiang, Bibbins-Domingo.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Kiang.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Kiang.
Administrative, technical, or material support: Carlasare, Thadaney-Israni, Norcini.
Supervision: Thadaney-Israni, Zaman.
Conflict of Interest Disclosures: Dr Kiang reported receiving grants from National Institute on Drug Abuse outside the submitted work. Ms Carlasare reported being employed by the American Medical Association. Ms Thadaney-Israni reported serving (unpaid) on the boards of UNICEF (Northwestern Regional Board), Scients, and the Society of Bedside Medicine. Dr Bibbins-Domingo reported being employed by the American Medical Association as the Editor in Chief of JAMA and the JAMA Network. No other disclosures were reported.
Additional Contributions: Isabella Chu, MPH, Deendayal Dinakarpandian, MD, PhD, and Abraham Verghese, MD, MACP, Stanford University, provided guidance and support, and Michael Tutty, PhD, MHA, and Tammy M. Weaver, BS, American Medical Association, shared their data expertise. These individuals received no compensation beyond their usual salaries for their contributions to this work.
Disclaimer: Dr Bibbins-Domingo is Editor in Chief of JAMA , but she was not involved in any of the decisions regarding review of the manuscript or its acceptance.
Data Sharing Statement: See Supplement 2 .
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
ORIGINAL RESEARCH article
Exploring behavioral intention to use telemedicine services post covid-19: a cross sectional study in saudi arabia.

- 1 Medical Informatics Unit, Medical Education Department, College of Medicine, King Saud Univeristy, Riyadh, Saudi Arabia
- 2 College of Medicine, King Saud University, Riyadh, Saudi Arabia
- 3 Department of Family and Community Medicine, College of Medicine, King Saud University, Riyadh, Saudi Arabia
- 4 College of Languages, Princess Nourah bint Abdulrahman University, Riyadh, Riyadh, Saudi Arabia
- 5 Evidence-Based Health Care & Knowledge Translation Research Chair, Department of Family & Community Medicine Department, King Saud University, Riyadh, Saudi Arabia
The final, formatted version of the article will be published soon.
Select one of your emails
You have multiple emails registered with Frontiers:
Notify me on publication
Please enter your email address:
If you already have an account, please login
You don't have a Frontiers account ? You can register here
While telemedicine offers significant benefits, there remain substantial knowledge gaps in the literature, particularly regarding its use in Saudi Arabia. This study aims to explore health consumers' behavioral intention to use telemedicine examining the associated factors such as eHealth literacy and attitudes toward telemedicine services. Methods: A cross-sectional observational study was conducted to collect data on demographics, health status, internet skills, attitudes toward telemedicine, and eHealth literacy. An online survey was administered at two large public gatherings in Riyadh. The eHEALS-Pl scale was used to measure perceived eHealth literacy levels, and data analysis was performed using SPSS (IBM Corp.Results: There were 385 participants, with an equal distribution of genders. The largest age group was 18-20 years old (57%). Nearly half of the participants were neither employed nor students, while 43% had access to governmental hospitals through employment. 71% reported proficiency in using the internet. Health-wise, 47% rated their health as excellent, and 56% did not have medical insurance.87% expressed a high likelihood of using telemedicine if offered by a provider. Participants were categorized based on their eHealth Literacy scores, with 54% scoring low and 46% scoring high. Overall, participants showed positive attitudes toward telemedicine, with 82% agreeing that it saves time, money, and provides access to specialized care. About half of the participants perceived the process of seeing a doctor through telemedicine video as complex. Both eHealth Literacy and attitudes toward telemedicine showed a statistically significant association with the intention to use telemedicine (p < 0.001). There was a positive and significant correlation between eHealth Literacy and attitudes (ρ =0.460; p < 0.001). Multivariate ordinal regression analysis revealed that the odds for a high likelihood of intention to use telemedicine significantly increased with positive attitudes (p < 0.001). Mediation analysis confirmed the significant mediating role of attitudes toward telemedicine in the relationship between eHealth Literacy and the intention to use telemedicine.The findings underline the importance of enhancing health literacy and consumer attitudes toward telemedicine, particularly during the healthcare digital transformation we are experiencing globally. This is crucial for promoting increased acceptance and utilization of telemedicine services beyond the COVID-19 pandemic.
Keywords: Telemedicine, attitudes, Behavioral Intention, EHealth literacy, Post COVID-19, Saudi Arabia
Received: 14 Feb 2024; Accepted: 05 Apr 2024.
Copyright: © 2024 Aldekhyyel, Alshuaibi, Alsaaid, Bin Moammar, Alanazy, Namshah, Altassan, Aldekhyyel and Jamal. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Raniah N. Aldekhyyel, Medical Informatics Unit, Medical Education Department, College of Medicine, King Saud Univeristy, Riyadh, Saudi Arabia
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.

IMAGES
COMMENTS
Coronavirus disease 2019 (COVID-19) is a highly contagious viral illness caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). COVID-19 has had a catastrophic effect on the world, resulting in more than 6 million deaths worldwide. After the first cases of this predominantly respiratory viral illness were reported in Wuhan, Hubei Province, China, in late December 2019, SARS ...
Explore a collection of articles and other resources on the Coronavirus (Covid-19) outbreak, including clinical reports, management guidelines, and commentary.
Coronavirus (COVID-19) research. Medical, social, and behavioral science articles from Sage Sage believes in the power of the social and behavioral sciences to convert the best medical research into policies, practices, and procedures to improve - and even save - lives. This collection includes the latest medical research from Sage related ...
A Crisis in Public Health. The United States has 4% of the world's population but, as of July 16, approximately 26% of its Covid-19 cases and 24% of its Covid-19 deaths. 17 These startling ...
Yet well into the fourth year of the Covid-19 pandemic, intense political and scientific debates about its origins continue. The two major hypotheses are a natural zoonotic spillover, most likely ...
2021 Top 25 COVID-19 Articles. The 25 most downloaded Nature Communications articles* on COVID-19 published in 2021 illustrate the collaborative efforts of the international community to combat ...
The research community has responded by publishing an impressive number of scientific reports related to COVID-19. The world was alerted to the new disease at the beginning of 2020 , and by mid-March 2020, more than 2000 articles had been published on COVID-19 in scholarly journals, with 25% of them containing original data .
In 2022, with increasing population level immunity, there will be substantial reductions in the number of people experiencing severe disease and death. This will alleviate some of the strain COVID ...
DOI: 10.1056/NEJMe2402224. Nirmatrelvir-ritonavir (Paxlovid [Pfizer]) is used as first-line therapy for nonhospitalized persons with Covid-19 1 on the basis of the results of the Evaluation of ...
The COVID-19 pandemic has developed for over one year. In many countries, repeated waves of COVID-19 have been observed. The primary aim of COVID-19 induced measures is to contain the virus by reducing physical contacts between people. Many of these measures immediately affect people's behaviors, but others could have longer-term impacts.
COVID-19: Uncovering Mental Health. Special issue of APA's journal Traumatology that includes fourteen articles detailing empirical and theoretical approaches seeking to gain a better understanding of how COVID-19 impacts our overall well-being, with a special focus on uncovering mental health disparities.
In the US, the COVID-19 pandemic has already resulted in over 1 million excess deaths, 1 defined as the difference between the number of observed and expected deaths over a specified period. 2 Despite their essential role in the pandemic response, little is known about excess deaths among physicians. Using data from the American Medical ...
A Global Natural Experiment. Over the past several months, the coronavirus disease 2019 (COVID-19) outbreak has rapidly grown to become a global pandemic. Countries, regions, and localities have taken varied and, in many cases, drastic approaches to limit the impact of COVID-19. Governments across the world have issued stay-at-home orders to ...
The RECOVERY Collaborative Group trial evaluated the efficacy of high and low doses of the anti-inflammatory corticosteroid dexamethasone in patients hospitalised for COVID-19 receiving oxygen or no oxygen with less than 92% saturation in room air.1 This trial was conducted with a major goal of establishing the efficacy of higher doses of dexamethasone treatment, which is a cost-effective ...
After the outbreak of the pandemic, research on COVID-19 has increased substantially. This study provides a literature review of COVID-19 research in the field of public administration. Applying a Structural Topic Model (STM), this review analyzes 710 articles. The analysis identifies and maps the 27 most salient topics.
VOL. 387 NO. 11. The coronavirus disease 2019 (Covid-19) pandemic has claimed an estimated 15 million lives, including more than 1 million lives in the United States alone. The rapid development ...
The public health response was successful in the suppression of COVID-19 transmission or the first half of 2020. However, a state election and outbreaks in institutionalised populations have been the catalyst for more significant community propagation. This rising community transmission has continued in 2021, leading to increased incidence and strained healthcare systems. Calibrating NPI based ...
Covid-19 vaccines were introduced at the participating facilities in December 2020, and the vaccine coverage among health care personnel at these facilities reached 55 to 98% for the receipt of at ...
This study addresses the knowledge gap on the impact of the COVID-19 pandemic ... Time to diagnosis (TTD) and treatment initiation (TTI) are important measures of access to and quality of cancer care. ... Journals metrics. This article was published in Asia Pacific Journal of Public Health. VIEW ALL JOURNAL METRICS. Article usage *
COVID-19 Resource Centre. As of January 2024, changes have been made to our COVID-19 Resource Centre. A COVID-19 Collection is available where you can continue to explore and access The Lancet Group's COVID-19 research, reviews, commentary, news, and analysis as it is published.
This article is part of the Research Topic Examining Upstream to Understand Downstream: Use of Telehealth and Other Health Equity Measures for Addressing Health Disparities View all 3 articles Exploring Behavioral Intention to Use Telemedicine Services Post COVID-19: A Cross Sectional Study in Saudi Arabia